Screening of Streptococcus pneumoniae ABC transporter mutants demonstrates that LivJHMGF, a branched-chain amino acid ABC transporter, is necessary for disease pathogenesis
- PMID: 19470745
- PMCID: PMC2715661
- DOI: 10.1128/IAI.01543-08
Screening of Streptococcus pneumoniae ABC transporter mutants demonstrates that LivJHMGF, a branched-chain amino acid ABC transporter, is necessary for disease pathogenesis
Abstract
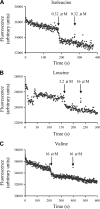
Bacterial ABC transporters are an important class of transmembrane transporters that have a wide variety of substrates and are important for the virulence of several bacterial pathogens, including Streptococcus pneumoniae. However, many S. pneumoniae ABC transporters have yet to be investigated for their role in virulence. Using insertional duplication mutagenesis mutants, we investigated the effects on virulence and in vitro growth of disruption of 9 S. pneumoniae ABC transporters. Several were partially attenuated in virulence compared to the wild-type parental strain in mouse models of infection. For one ABC transporter, required for full virulence and termed LivJHMGF due to its similarity to branched-chain amino acid (BCAA) transporters, a deletion mutant (DeltalivHMGF) was constructed to investigate its phenotype in more detail. When tested by competitive infection, the DeltalivHMGF strain had reduced virulence in models of both pneumonia and septicemia but was fully virulent when tested using noncompetitive experiments. The DeltalivHMGF strain had no detectable growth defect in defined or complete laboratory media. Recombinant LivJ, the substrate binding component of the LivJHMGF, was shown by both radioactive binding experiments and tryptophan fluorescence spectroscopy to specifically bind to leucine, isoleucine, and valine, confirming that the LivJHMGF substrates are BCAAs. These data demonstrate a previously unsuspected role for BCAA transport during infection for S. pneumoniae and provide more evidence that functioning ABC transporters are required for the full virulence of bacterial pathogens.
Figures




References
-
- Adams, M. D., L. M. Wagner, T. J. Graddis, R. Landick, T. K. Antonucci, A. L. Gibson, and D. L. Oxender. 1990. Nucleotide sequence and genetic characterization reveal six essential genes for the LIV-I and LS transport systems of Escherichia coli. J. Biol. Chem. 26511436-11443. - PubMed
-
- Anderton, J. M., G. Rajam, S. Romero-Steiner, S. Summer, A. P. Kowalczyk, G. M. Carlone, J. S. Sampson, and E. W. Ades. 2007. E-cadherin is a receptor for the common protein pneumococcal surface adhesin A (PsaA) of Streptococcus pneumoniae. Microb. Pathog. 42225-236. - PubMed
-
- Atkins, T., R. G. Prior, K. Mack, P. Russell, M. Nelson, P. C. Oyston, G. Dougan, and R. W. Titball. 2002. A mutant of Burkholderia pseudomallei, auxotrophic in the branched chain amino acid biosynthetic pathway, is attenuated and protective in a murine model of melioidosis. Infect. Immun. 705290-5294. - PMC - PubMed
-
- Bergmann, S., and S. Hammerschmidt. 2006. Versatility of pneumococcal surface proteins. Microbiology 152295-303. - PubMed
-
- Beuzon, C. R., and D. W. Holden. 2001. Use of mixed infections with Salmonella strains to study virulence genes and their interactions in vivo. Microbes Infect. 31345-1352. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

