How does treatment satisfaction work?: Modeling determinants of treatment satisfaction and preference
- PMID: 19470837
- PMCID: PMC2713611
- DOI: 10.2337/dc08-2256
How does treatment satisfaction work?: Modeling determinants of treatment satisfaction and preference
Abstract
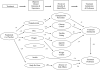
OBJECTIVE This study tested a model hypothesizing that treatment affects objective clinical outcomes, which in turn affect perceived consequences, which in turn affect satisfaction and preference judgments. RESEARCH DESIGN AND METHODS The model was tested in a double-blind, randomized clinical trial in which 266 patients with type 1 diabetes added active or placebo pramlintide to their insulin regimens. Objective clinical outcomes included changes in glucose and weight control, insulin requirements, incidence of hypoglycemia, and study drug tolerance. At the end of the trial, patients completed the validated PRAM-TSQ questionnaire measuring treatment satisfaction and preference and perceived medication benefits and side effects. RESULTS Statistical modeling demonstrated that active pramlintide was significantly associated with greater treatment satisfaction, preference, and perceived benefits (all except hypoglycemia prevention), as well as objective clinical outcomes (weight loss, lower postprandial glucose [PPG], lower medication tolerance, more hypoglycemia). Perceptions of treatment consequences were sensitive and specific to their cognate objective clinical outcomes (no halo effects). Clinical outcomes (especially PPG) accounted for almost half of the effect of the study medication on treatment satisfaction and preference. Treatment satisfaction and preference were strongly related to the perceived benefits/side effects of the study medication, and these perceptions (especially glucose control) mediated most of the association of clinical outcomes with satisfaction and preference. CONCLUSIONS This model received substantial empirical support. Improvements in objective clinical outcomes accounted for a large part of the association of pramlintide treatment with higher treatment satisfaction and preference. Perceived treatment consequences mediated the effect of objective clinical benefits on satisfaction with and preference for the study medication.
Figures
References
-
- Food and Drug Administration. Guidance for industry: patient-reported outcome measures: use in medical product development and labeling claims. Available at http://www.fda.gov/cder/guidance/5460dft.pdf. Accessed 24 March 2008
-
- Marra G. the DIAB.&TE.S Project Study Group. The DIAB. &TE.S Project: how patients perceive diabetes and diabetes therapy. Acta Biomed 2004; 75: 164– 170 - PubMed
-
- Charpentier G, Fleury F, Dubroca I, Vaur L, Clerson P: Electronic pill-boxes in the evaluation of oral hypoglycemic agent compliance. Diabetes Metab 2005; 31: 189– 195 - PubMed
-
- Kelley K, Dempsey C: An evaluation of an insulin transfer programme delivered in a group setting. J Clin Nurs 2007; 16: 152– 158 - PubMed
-
- Tahrani AA, Digwood S, Lee C, Moulilk P: Evaluation of glargine group-start sessions in patients with type 2 diabetes as a strategy to deliver the service. Int J Clin Pract 2007; 61: 329– 335 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

