Vascular regeneration by local growth factor release is self-limited by microvascular clearance
- PMID: 19470891
- PMCID: PMC2764268
- DOI: 10.1161/CIRCULATIONAHA.108.823609
Vascular regeneration by local growth factor release is self-limited by microvascular clearance
Abstract
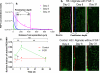
Background: The challenge of angiogenesis science is that stable sustained vascular regeneration in humans has not been realized despite promising preclinical findings. We hypothesized that angiogenic therapies powerfully self-regulate by dynamically altering tissue characteristics. Induced neocapillaries increase drug clearance and limit tissue retention and subsequent angiogenesis even in the face of sustained delivery.
Methods and results: We quantified how capillary flow clears fibroblast growth factor after local epicardial delivery. Fibroblast growth factor spatial loading was significantly reduced with intact coronary perfusion. Penetration and retention decreased with transendothelial permeability, a trend diametrically opposite to intravascular delivery, in which factor delivery depends on vascular leak, but consistent with a continuum model of drug transport in perfused tissues. Model predictions of fibroblast growth factor sensitivity to manipulations of its diffusivity and transendothelial permeability were validated by conjugation to sucrose octasulfate. Induction of neocapillaries adds pharmacokinetic complexity. Sustained local fibroblast growth factor delivery in vivo produced a burst of neovascularization in ischemic myocardium but was followed by drug washout and a 5-fold decrease in fibroblast growth factor penetration depth.
Conclusions: The very efficacy of proangiogenic compounds enhances their clearance and abrogates their pharmacological benefit. This self-limiting property of angiogenesis may explain the failures of promising proangiogenic therapies.
Figures




References
-
- Yanagisawa-Miwa A, Uchida Y, Nakamura F, Tomaru T, Kido H, Kamijo T, Sugimoto T, Kaji K, Utsuyama M, Kurashima C. Salvage of infarcted myocardium by angiogenic action of basic fibroblast growth factor. Science. 1992;257:1401–1403. - PubMed
-
- Takeshita S, Pu LQ, Stein LA, Sniderman AD, Bunting S, Ferrara N, Isner JM, Symes JF. Intramuscular administration of vascular endothelial growth factor induces dose-dependent collateral artery augmentation in a rabbit model of chronic limb ischemia. Circulation. 1994;90:II228–234. - PubMed
-
- Harada K, Friedman M, Lopez JJ, Wang SY, Li J, Prasad PV, Pearlman JD, Edelman ER, Sellke FW, Simons M. Vascular endothelial growth factor administration in chronic myocardial ischemia. Am J Physiol. 1996;270:H1791–1802. - PubMed
-
- Lopez JJ, Edelman ER, Stamler A, Hibberd MG, Prasad P, Thomas KA, DiSalvo J, Caputo RP, Carrozza JP, Douglas PS, Sellke FW, Simons M. Angiogenic potential of perivascularly delivered aFGF in a porcine model of chronic myocardial ischemia. Am J Physiol. 1998;274:H930–936. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

