Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study
- PMID: 19470923
- PMCID: PMC2702235
- DOI: 10.1200/JCO.2008.20.9908
Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: a phase II study
Abstract
Purpose: To assess the safety and efficacy of sunitinib in patients with advanced hepatocellular carcinoma (HCC) and explore biomarkers for sunitinib response.
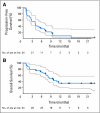
Patients and methods: We conducted a multidisciplinary phase II study of sunitinib, an antivascular endothelial growth factor receptor tyrosine kinase inhibitor, in advanced HCC. Patients received sunitinib 37.5 mg/d for 4 weeks followed by 2 weeks of rest per cycle. The primary end point was progression-free survival (PFS). We used functional magnetic resonance imaging to evaluate vascular changes in HCC after sunitinib treatment. Circulating molecular and cellular biomarkers were evaluated before and at six time points after sunitinib treatment.
Results: Thirty-four patients were enrolled. The objective response rate was 2.9%, and 50% of patients had stable disease. Median PFS was 3.9 months (95% CI, 2.6 to 6.9 months), and overall survival was 9.8 months (95% CI, 7.4 months to not available). Grade 3 or 4 toxicities included leukopenia/neutropenia, thrombocytopenia, elevation of aminotransferases, and fatigue. Sunitinib rapidly decreased vessel leakiness, and this effect was more pronounced in patients with delayed progression. When evaluated early (at baseline and day 14) as well as over three cycles of treatment, higher levels of inflammatory molecules (eg, interleukin-6, stromal-derived factor 1alpha, soluble c-KIT) and circulating progenitor cells were associated with a poor outcome.
Conclusion: Sunitinib shows evidence of modest antitumor activity in advanced HCC with manageable adverse effects. Rapid changes in tumor vascular permeability and circulating inflammatory biomarkers are potential determinants of response and resistance to sunitinib in HCC. Our study suggests that control of inflammation might be critical for improving treatment outcome in advanced HCC.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest and author contributions are found at the end of this article.
Figures


Comment in
-
Sunitinib in hepatocellular carcinoma: redefining appropriate dosing, schedule, and activity end points.J Clin Oncol. 2009 Dec 10;27(35):e248-50; author reply e251-2. doi: 10.1200/JCO.2009.25.0670. Epub 2009 Nov 9. J Clin Oncol. 2009. PMID: 19901099 No abstract available.
References
-
- Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. - PubMed
-
- El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. - PubMed
-
- McGlynn KA, London WT. Epidemiology and natural history of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol. 2005;19:3–23. - PubMed
-
- Zhu AX. Systemic therapy of advanced hepatocellular carcinoma: How hopeful should we be? Oncologist. 2006;11:790–800. - PubMed
-
- de Visser KE, Eichten A, Coussens LM. Paradoxical roles of the immune system during cancer development. Nat Rev Cancer. 2006;6:24–37. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

