Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer
- PMID: 19470933
- PMCID: PMC3535569
- DOI: 10.1200/JCO.2008.20.0642
Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer
Abstract
Purpose: It has been postulated that castration-resistant prostate cancer (CRPC) commonly remains hormone dependent. Abiraterone acetate is a potent, selective, and orally available inhibitor of CYP17, the key enzyme in androgen and estrogen biosynthesis.
Patients and methods: This was a phase I/II study of abiraterone acetate in castrate, chemotherapy-naive CRPC patients (n = 54) with phase II expansion at 1,000 mg (n = 42) using a two-stage design to reject the null hypothesis if more than seven patients had a prostate-specific antigen (PSA) decline of > or = 50% (null hypothesis = 0.1; alternative hypothesis = 0.3; alpha = .05; beta = .14). Computed tomography scans every 12 weeks and circulating tumor cell (CTC) enumeration were performed. Prospective reversal of resistance at progression by adding dexamethasone 0.5 mg/d to suppress adrenocorticotropic hormone and upstream steroids was pursued.
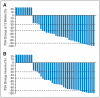
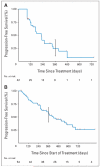
Results: A decline in PSA of > or = 50% was observed in 28 (67%) of 42 phase II patients, and declines of > or = 90% were observed in eight (19%) of 42 patients. Independent radiologic evaluation reported partial responses (Response Evaluation Criteria in Solid Tumors) in nine (37.5%) of 24 phase II patients with measurable disease. Decreases in CTC counts were also documented. The median time to PSA progression (TTPP) on abiraterone acetate alone for all phase II patients was 225 days (95% CI, 162 to 287 days). Exploratory analyses were performed on all 54 phase I/II patients; the addition of dexamethasone at disease progression reversed resistance in 33% of patients regardless of prior treatment with dexamethasone, and pretreatment serum androgen and estradiol levels were associated with a probability of > or = 50% PSA decline and TTPP on abiraterone acetate and dexamethasone.
Conclusion: CYP17 blockade by abiraterone acetate results in declines in PSA and CTC counts and radiologic responses, confirming that CRPC commonly remains hormone driven.
Figures

Comment in
-
Words of wisdom. Re: Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer.Eur Urol. 2009 Oct;56(4):744-5. doi: 10.1016/j.eururo.2009.07.012. Eur Urol. 2009. PMID: 19995526 No abstract available.
References
-
- Prostate Cancer Trialists’ Collaborative Group Maximum androgen blockade in advanced prostate cancer: An overview of the randomised trials. Lancet. 2000;355:1491–1498. - PubMed
-
- Hellerstedt BA, Pienta KJ. The current state of hormonal therapy for prostate cancer. CA Cancer J Clin. 2002;52:154–179. - PubMed
-
- Attard G, Belldegrun AS, de Bono JS. Selective blockade of androgenic steroid synthesis by novel lyase inhibitors as a therapeutic strategy for treating metastatic prostate cancer. BJU Int. 2005;96:1241–1246. - PubMed
-
- Labrie F, Dupont A, Belanger A, et al. New hormonal therapy in prostatic carcinoma: Combined treatment with an LHRH agonist and an antiandrogen. Clin Invest Med. 1982;5:267–275. - PubMed
-
- Mohler JL, Gregory CW, Ford OH, 3rd, et al. The androgen axis in recurrent prostate cancer. Clin Cancer Res. 2004;10:440–448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

