The role of the tripartite glutamatergic synapse in the pathophysiology and therapeutics of mood disorders
- PMID: 19471044
- PMCID: PMC2762009
- DOI: 10.1177/1073858409336093
The role of the tripartite glutamatergic synapse in the pathophysiology and therapeutics of mood disorders
Erratum in
- Neuroscientist. 2010 Apr;16(2):199
Abstract
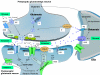
Bipolar disorder and major depressive disorder are common, chronic, and recurrent mood disorders that affect the lives of millions of individuals worldwide. Growing evidence suggests that glutamatergic system dysfunction is directly involved in mood disorders. This article describes the role of the "tripartite glutamatergic synapse," comprising presynaptic and postsynaptic neurons and glial cells, in the pathophysiology and therapeutics of mood disorders. Glutamatergic neurons and glia directly control synaptic and extrasynaptic glutamate levels/ release through integrative effects that target glutamate excitatory amino acid transporters, postsynaptic density proteins, ionotropic receptors (alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid [AMPA], N-methyl-D-aspartate [NMDA], and kainate), and metabotropic receptors. This article also explores the glutamatergic modulators riluzole and ketamine, which are considered valuable proof-of-concept agents for developing the next generation of antidepressants and mood stabilizers. In therapeutically relevant paradigms, ketamine preferentially targets postsynaptic AMPA/NMDA receptors, and riluzole preferentially targets presynaptic voltage-operated channels and glia.
Figures
References
-
- Aguado L, San Antonio A, Perez L, del Valle R, Gomez J. Effects of the NMDA receptor antagonist ketamine on flavor memory: conditioned aversion, latent inhibition, and habituation of neophobia. Behav Neural Biol. 1994;61:271–81. - PubMed
-
- Altamura CA, Mauri MC, Ferrara A, Moro AR, D’Andrea G, Zamberlan F. Plasma and platelet excitatory amino acids in psychiatric disorders. Am J Psychiatry. 1993;150:1731–3. - PubMed
-
- Banasr M, Valentine GW, Li XY, Gourley SL, Taylor JR, Duman RS. Chronic unpredictable stress decreases cell proliferation in the cerebral cortex of the adult rat. Biol Psychiatry. 2007;62:496–504. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

