Plasma proteomic analysis of patients with squamous cell carcinoma of the uterine cervix
- PMID: 19471570
- PMCID: PMC2676467
- DOI: 10.3802/jgo.2008.19.3.173
Plasma proteomic analysis of patients with squamous cell carcinoma of the uterine cervix
Abstract
Objective: To compare plasma protein expression between patients with squamous cell carcinoma (SCC) of the cervix and normal controls.
Methods: Plasma samples from patients with benign gynecological disease (normal cervix, n=6) and cervical cancer (SCC, n=6) were subjected to plasma proteomic analysis using two dimensional gel electrophoresis (2-DE) and matrix-assisted laser desorption/ionization mass spectroscopy (MALDI-MS). Western blotting and immunoturbidimetric assay were performed to validate the results of 2-DE.
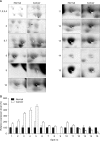
Results: Eight proteins showed differential expression between controls and SCC patients; six (ceruloplasmin, complement C3, afamin precursor, alpha-1-B-glycoprotein, transferrin, alpha-fibrinogen precursor) were up-regulated, while two (chain A, crystal structure of antithrombin and apolipoprotein A-IV precursor) were down-regulated in the plasma of SCC patients. Western blotting analysis revealed significant elevation of ceruloplasmin, complement C3, afamin, and alpha-1-B-glycoprotein in the plasma of SCC patients in comparison to controls. Immunoturbidimetric assay of a larger group confirmed the results of 2-DE and Western blotting, and showed that ceruloplasmin and complement C3 were significantly elevated in the plasma of SCC patients in comparison with controls and patients with carcinoma in situ (CIS) of the uterine cervix.
Conclusion: Plasma protein expression determined using 2-DE and MALDI-MS will give a chance to identify tumor-specific biomarkers for SCC of the cervix.
Keywords: MALDI-MS; Plasma proteins; Squamous cell carcinoma; Two dimensional gel electrophoresis; Uterine cervical cancer.
Figures


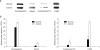
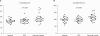
Similar articles
-
Two-dimensional gel analysis of protein expression profile in squamous cervical cancer patients.Gynecol Oncol. 2005 Oct;99(1):26-35. doi: 10.1016/j.ygyno.2005.05.041. Gynecol Oncol. 2005. PMID: 16051329
-
Protein expression profile using two-dimensional gel analysis in squamous cervical cancer patients.Cancer Res Treat. 2006 Apr;38(2):99-107. doi: 10.4143/crt.2006.38.2.99. Epub 2006 Apr 30. Cancer Res Treat. 2006. PMID: 19771267 Free PMC article.
-
[Expression and clinical significance of AHSG and complement C3 in pancreatic ductal adenocarcinoma].Zhonghua Yi Xue Za Zhi. 2014 Jul 22;94(28):2175-9. Zhonghua Yi Xue Za Zhi. 2014. PMID: 25331466 Chinese.
-
Factors regulating SCC antigen expression in squamous cell carcinoma of the uterine cervix.Tumour Biol. 1998;19(6):494-504. doi: 10.1159/000030043. Tumour Biol. 1998. PMID: 9817979 Review.
-
Co-existing adenoid cystic carcinoma and invasive squamous cell carcinoma of the uterine cervix: a rare case report and literature review.Ann Clin Lab Sci. 2014 Fall;44(4):502-7. Ann Clin Lab Sci. 2014. PMID: 25361940 Review.
Cited by
-
Role of C5b-9 complement complex and response gene to complement-32 (RGC-32) in cancer.Immunol Res. 2013 May;56(1):109-21. doi: 10.1007/s12026-012-8381-8. Immunol Res. 2013. PMID: 23247987 Review.
-
Multi-omics differences in the bone marrow between essential thrombocythemia and prefibrotic primary myelofibrosis.Clin Exp Med. 2024 Jul 8;24(1):154. doi: 10.1007/s10238-024-01350-y. Clin Exp Med. 2024. PMID: 38972952 Free PMC article.
-
Evaluation of potential anti-metastatic and antioxidative abilities of natural peptides derived from Tecoma stans (L.) Juss. ex Kunth in A549 cells.PeerJ. 2022 Jul 6;10:e13693. doi: 10.7717/peerj.13693. eCollection 2022. PeerJ. 2022. PMID: 35818360 Free PMC article.
-
Proteomic Analysis Identifies FNDC1, A1BG, and Antigen Processing Proteins Associated with Tumor Heterogeneity and Malignancy in a Canine Model of Breast Cancer.Cancers (Basel). 2021 Nov 24;13(23):5901. doi: 10.3390/cancers13235901. Cancers (Basel). 2021. PMID: 34885011 Free PMC article.
-
A novel electrochemical immunosensor for sensitive detection of depression marker Apo-A4 based on bipyridine-functionalized covalent organic frameworks.Mikrochim Acta. 2024 Mar 5;191(4):179. doi: 10.1007/s00604-024-06260-0. Mikrochim Acta. 2024. PMID: 38443677
References
-
- Parkin DM, Bray F, Ferlay J, Pisani P. Global cancer statistics, 2002. CA Cancer J Clin. 2005;55:74–108. - PubMed
-
- National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Fort Washington: National Comprehensive Cancer Network; 2008.
-
- Liao SY, Stanbridge EJ. Expression of MN/CA9 protein in Papanicolaou smears containing atypical glandular cells of undetermined significance is a diagnostic biomarker of cervical dysplasia and neoplasia. Cancer. 2000;88:1108–1121. - PubMed
-
- Kurman RJ, Henson DE, Herbst AL, Noller KL, Schiffman MH. Interim guidelines for management of abnormal cervical cytology: The 1992 National Cancer Institute Workshop. JAMA. 1994;271:1866–1869. - PubMed
-
- Koss LG. Cervical (Pap) smear: New directions. Cancer. 1993;71(4 Suppl):1406–1412. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

