The treatment of colorectal carcinoma with monoclonal antibodies: the importance of KRAS mutation analysis and EGFR status
- PMID: 19471640
- PMCID: PMC2680580
- DOI: 10.3238/arztebl.2009.0202
The treatment of colorectal carcinoma with monoclonal antibodies: the importance of KRAS mutation analysis and EGFR status
Abstract
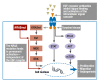
Background: The epidermal growth factor receptor (EGFR) is an important target in the treatment of metastatic colorectal carcinoma (mCRC). The combination of anti-EGFR antibodies with chemotherapy has led to a higher response rate of certain kinds of tumor as well as a significant prolongation of the progression-free interval. The KRAS protein is an important mediator in the signal transduction cascade regulated by the EGFR. A KRAS mutation is present in 30% to 49% of all colorectal carcinomas. Mutations in the KRAS gene can be demonstrated by the methods of molecular pathology and are a very important factor in the selection of molecular biological treatment options targeted against EGFR.
Methods: Selective literature review.
Results: Patients bearing mutations of the KRAS gene do not benefit from treatment with the EGFR antibodies cetuximab and panitumumab.
Conclusions: Activating mutations of the KRAS gene are biomarkers for resistance to cetuximab or panitumumab. Thus, anti-EGFR therapies are approved for the treatment of metastatic colorectal carcinoma only on condition that the mutation state of the KRAS gene is determined first, because the combination of chemotherapy with anti-EGFR is expected to increase the response rate only in patients with the wild-type KRAS gene.
Keywords: cancer therapy; cetuximab; colorectal carcinoma; gene mutation; molecular medicine.
Figures
Comment in
-
Subsequent Immunological Mechanisms.Dtsch Arztebl Int. 2009 Jul;106(31-32):526; author reply 526. doi: 10.3238/arztebl.2009.0526a. Epub 2009 Aug 3. Dtsch Arztebl Int. 2009. PMID: 19730726 Free PMC article. No abstract available.
References
-
- Cunningham D, et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N Engl J Med. 2004;351:337–345. - PubMed
-
- Mayer A, et al. The prognostic significance of proliferating cell nuclear antigen, epidermal growth factor receptor, and mdr gene expression in colorectal cancer. Cancer. 1993;71:2454–2460. - PubMed
-
- Nicholson RI, Gee JM, Harper ME. EGFR and cancer prognosis. Eur J Cancer. 2001;37:9–15. - PubMed
-
- Saltz LB, et al. Phase II trial of cetuximab in patients with refractory colorectal cancer that expresses the epidermal growth factor receptor. J Clin Oncol. 2004;22:1201–1208. - PubMed
-
- Mendelsohn J, Baselga J. Status of epidermal growth factor receptor antagonists in the biology and treatment of cancer. J Clin Oncol. 2003;21:2787–2799. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous