Hydrogels as extracellular matrix mimics for 3D cell culture
- PMID: 19472329
- PMCID: PMC2997742
- DOI: 10.1002/bit.22361
Hydrogels as extracellular matrix mimics for 3D cell culture
Abstract
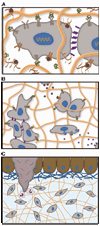
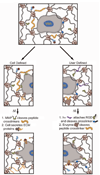
Methods for culturing mammalian cells ex vivo are increasingly needed to study cell and tissue physiology and to grow replacement tissue for regenerative medicine. Two-dimensional culture has been the paradigm for typical in vitro cell culture; however, it has been demonstrated that cells behave more natively when cultured in three-dimensional environments. Permissive, synthetic hydrogels and promoting, natural hydrogels have become popular as three-dimensional cell culture platforms; yet, both of these systems possess limitations. In this perspective, we discuss the use of both synthetic and natural hydrogels as scaffolds for three-dimensional cell culture as well as synthetic hydrogels that incorporate sophisticated biochemical and mechanical cues as mimics of the native extracellular matrix. Ultimately, advances in synthetic-biologic hydrogel hybrids are needed to provide robust platforms for investigating cell physiology and fabricating tissue outside of the organism.
(c) 2009 Wiley Periodicals, Inc.
Figures


References
-
- Ashe HL, Briscoe J. The interpretation of morphogen gradients. Development. 2006;133(3):385–394. - PubMed
-
- Azab AK, Orkin B, Doviner V, Nissan A, Klein M, Srebnik M, Rubinstein A. Crosslinked chitosan implants as potential degradable devices for brachytherapy: In vitro and in vivo analysis. J Control Release. 2006;111(3):281–289. - PubMed
-
- Barralet JE, Wang L, Lawson M, Triffitt JT, Cooper PR, Shelton RM. Comparison of bone marrow cell growth on 2D and 3D alginate hydrogels. J Mater Sci Mater Med. 2005;16:515–519. - PubMed
-
- Birgersdotter A, Sandberg R, Ernberg I. Gene expression perturbation in vitro—A growing case for three-dimensional (3D) culture systems. Semin Cancer Biol. 2005;15(5):405–412. - PubMed
-
- Bryant SJ, Anseth KS. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J Biomed Mater Res. 2002;59(1):63–72. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

