NV-128, a novel isoflavone derivative, induces caspase-independent cell death through the Akt/mammalian target of rapamycin pathway
- PMID: 19472400
- PMCID: PMC2757274
- DOI: 10.1002/cncr.24397
NV-128, a novel isoflavone derivative, induces caspase-independent cell death through the Akt/mammalian target of rapamycin pathway
Abstract
Background: Resistance to apoptosis is 1 of the key events that confer chemoresistance and is mediated by the overexpression of antiapoptotic proteins, which inhibit caspase activation. The objective of this study was to evaluate whether the activation of an alternative, caspase-independent cell death pathway could promote death in chemoresistant ovarian cancer cells. The authors report the characterization of NV-128 as an inducer of cell death through a caspase-independent pathway.
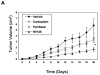
Methods: Primary cultures of epithelial ovarian cancer (EOC) cells were treated with increasing concentration of NV-128, and the concentration that caused 50% growth inhibition (GI(50)) was determined using a proprietary assay. Apoptotic proteins were characterized by Western blot analyses, assays that measured caspase activity, immunohistochemistry, and flow cytometry. Protein-protein interactions were determined using immunoprecipitation. In vivo activity was measured in a xenograft mice model.
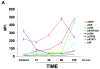
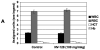
Results: NV-128 was able to induce significant cell death in both paclitaxel-resistant and carboplatin-resistant EOC cells with a GI(50) between 1 microg/mL and 5 microg/mL. Cell death was characterized by chromatin condensation but was caspase-independent. The activated pathway involved the down-regulation of phosphorylated AKT, phosphorylated mammalian target of rapamycin (mTOR), and phosphorylated ribosomal p70 S6 kinase, and the mitochondrial translocation of beclin-1 followed by nuclear translocation of endonuclease G.
Conclusions: The authors characterized a novel compound, NV-128, which inhibits mTOR and promotes caspase-independent cell death. The current results indicated that inhibition of mTOR may represent a relevant pathway for the induction of cell death in cells resistant to the classic caspase-dependent apoptosis. These findings demonstrate the possibility of using therapeutic drugs, such as NV-128, which may have beneficial effects in patients with chemoresistant ovarian cancer.
Figures




















References
-
- Schwartz PE. Current diagnosis and treatment modalities for ovarian cancer. Cancer Treat Res. 2002;107:99–118. - PubMed
-
- Igney FH, Krammer PH. Death and anti-death: tumour resistance to apoptosis. Nat Rev Cancer. 2002;2:277–288. - PubMed
-
- Edinger AL, Thompson CB. Death by design: apoptosis, necrosis and autophagy. Curr Opin Cell Biol. 2004;16:663–669. - PubMed
-
- Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281:1305–1308. - PubMed
-
- Cory S, Adams JM. The Bcl2 family: regulators of the cellular life-or-death switch. Nat Rev Cancer. 2002;2:647–656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

