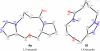Accessing skeletal diversity using catalyst control: formation of n and n + 1 macrocyclic triazole rings
- PMID: 19473044
- PMCID: PMC2702139
- DOI: 10.1021/ol900562u
Accessing skeletal diversity using catalyst control: formation of n and n + 1 macrocyclic triazole rings
Abstract
A regioselective intramolecular Huisgen cycloaddition was performed on various azido alkyne substrates giving rise to macrocyclic triazole rings. Using catalyst control, a common intermediate has been converted to two structurally unique macrocycles with either a 1,5- or a 1,4-triazole resulting in an n or n + 1 ring size. This is the first example of an intramolecular ruthenium-catalyzed Huisgen cycloaddition.
Figures
Similar articles
-
A recyclable ruthenium(II) complex supported on magnetic nanoparticles: a regioselective catalyst for alkyne-azide cycloaddition.Chem Commun (Camb). 2013 Aug 11;49(62):6956-8. doi: 10.1039/c3cc43048k. Chem Commun (Camb). 2013. PMID: 23807317
-
A concomitant allylic azide rearrangement/intramolecular azide-alkyne cycloaddition sequence.Org Lett. 2014 Apr 4;16(7):1844-7. doi: 10.1021/ol500011f. Epub 2014 Mar 17. Org Lett. 2014. PMID: 24635056 Free PMC article.
-
Zinc mediated azide-alkyne ligation to 1,5- and 1,4,5-substituted 1,2,3-triazoles.Org Lett. 2013 Sep 20;15(18):4826-9. doi: 10.1021/ol402225d. Epub 2013 Sep 3. Org Lett. 2013. PMID: 24001177 Free PMC article.
-
A New Insight Into The Huisgen Reaction: Heterogeneous Copper Catalyzed Azide-Alkyne Cycloaddition for the Synthesis of 1,4-Disubstituted Triazole (From 2018-2023).Chem Biodivers. 2024 Jun;21(6):e202400109. doi: 10.1002/cbdv.202400109. Epub 2024 May 22. Chem Biodivers. 2024. PMID: 38640439 Review.
-
Decarboxylative click cycloaddition: an emerging strategy towards substituted 1,2,3-triazole derivatives.Mol Divers. 2025 Jun;29(3):2811-2827. doi: 10.1007/s11030-024-11014-4. Epub 2024 Nov 10. Mol Divers. 2025. PMID: 39522072 Review.
Cited by
-
Recent Progress on Synthesis of Functionalized 1,5-Disubstituted Triazoles.Curr Org Synth. 2024;21(4):513-558. doi: 10.2174/1570179420666230418123350. Curr Org Synth. 2024. PMID: 38804327 Review.
-
Regioselective intramolecular dipolar cycloaddition of azides and unsymmetrical alkynes.Org Lett. 2010 Jan 15;12(2):336-9. doi: 10.1021/ol902681t. Org Lett. 2010. PMID: 20017521 Free PMC article.
-
Insights on the Synthesis of N-Heterocycles Containing Macrocycles and Their Complexion and Biological Properties.Molecules. 2022 Mar 25;27(7):2123. doi: 10.3390/molecules27072123. Molecules. 2022. PMID: 35408522 Free PMC article. Review.
-
A diversity-oriented synthesis strategy enabling the combinatorial-type variation of macrocyclic peptidomimetic scaffolds.Org Biomol Chem. 2015 Apr 21;13(15):4570-80. doi: 10.1039/c5ob00371g. Org Biomol Chem. 2015. PMID: 25778821 Free PMC article.
-
A strategy for the diversity-oriented synthesis of macrocyclic scaffolds using multidimensional coupling.Nat Chem. 2013 Oct;5(10):861-7. doi: 10.1038/nchem.1729. Epub 2013 Aug 25. Nat Chem. 2013. PMID: 24056343
References
-
- Burke MD, Schreiber SL. Agnew. Chem. Int. Ed. 2004;43:5178. - PubMed
-
- Burke MD, Berger EM, Schreiber SL. Science. 2003;302:613. - PubMed
-
- Ortholand J, Ganesan A. Curr. Opin. Chem. Bio. 2004;8:270. - PubMed
-
- Payne DJ, Gwynn MN, Holmes DJ, Pompliano DL. Nature Reviews Drug Discovery. 2007;6:29. - PubMed
-
- Comer E, Rohan E, Deng L, Porco JA. Org. Lett. 2007;9:2123–2126. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources