A pilot trial of serial 18F-fluorodeoxyglucose positron emission tomography in patients with medically inoperable stage I non-small-cell lung cancer treated with hypofractionated stereotactic body radiotherapy
- PMID: 19473777
- PMCID: PMC2823932
- DOI: 10.1016/j.ijrobp.2009.02.051
A pilot trial of serial 18F-fluorodeoxyglucose positron emission tomography in patients with medically inoperable stage I non-small-cell lung cancer treated with hypofractionated stereotactic body radiotherapy
Abstract
Purpose: Routine assessment was made of tumor metabolic activity as measured by 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) in Stage I non-small-cell lung cancer (NSCLC). This report describes PET correlates prospectively collected after stereotactic body radiotherapy (SBRT) for patients with medically inoperable NSCLC.
Methods and materials: 14 consecutive patients with medically inoperable Stage I NSCLC were enrolled. All patients received SBRT to 60-66 Gy in three fractions. Patients underwent serial planned FDG-PET/computed tomography fusion imaging before SBRT and at 2, 26, and 52 weeks after SBRT.
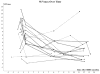
Results: With median follow-up of 30.2 months, no patients experienced local failure. One patient developed regional failure, 1 developed distant failure, and 1 developed a second primary. The median tumor maximum standardized uptake value (SUV(max)) before SBRT was 8.70. The median SUV(max) values at 2, 26, and 52 weeks after SBRT were 6.04, 2.80, and 3.58, respectively. Patients with low pre-SBRT SUV were more likely to experience initial 2-week rises in SUV, whereas patients with high pre-SBRT SUV commonly had SUV declines 2 weeks after treatment (p = 0.036). Six of 13 patients had primary tumor SUV(max) >3.5 at 12 months after SBRT but remained without evidence of local disease failure on further follow-up.
Conclusions: A substantial proportion of patients may have moderately elevated FDG-PET SUV(max) at 12 months without evidence of local failure on further follow-up. Thus, slightly elevated PET SUV(max) should not be considered a surrogate for local treatment failure. Our data do not support routine serial FDG-PET/computed tomography for follow-up of patients receiving SBRT for Stage I NSCLC.
Copyright (c) 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
Mark Henderson: no financial or other actual or potential conflicts of interest exist.
David Hoopes: no financial or other actual or potential conflicts of interest exist.
James Fletcher: no financial or other actual or potential conflicts of interest exist.
Pei-Fin Lin: no financial or other actual or potential conflicts of interest exist.
Mark Tann: no financial or other actual or potential conflicts of interest exist.
Constantin Yiannoutsos: no financial or other actual or potential conflicts of interest exist.
Mark Williams: no financial or other actual or potential conflicts of interest exist.
Achilles Fakiris: no financial or other actual or potential conflicts of interest exist.
Ronald McGarry: no financial or other actual or potential conflicts of interest exist.
Robert Timmerman: no financial or other actual or potential conflicts of interest exist.
No copyrighted information or patient photos were used.
Figures
References
-
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. - PubMed
-
- Martini N, Bains MS, Burt ME, et al. Incidence of local recurrence and second primary tumors in resected stage I lung cancer. J Thorac Cardiovasc Surg. 1995;109:120–129. - PubMed
-
- Adebonojo SA, Bowser AN, Moritz DM, et al. Impact of revised stage classification of lung cancer on survival: a military experience. Chest. 1999;115:1507–1513. - PubMed
-
- Mountain CF. A new international staging system for lung cancer. Chest. 1986;89:225S–233S. - PubMed
-
- Naruke T, Goya T, Tsuchiya R, et al. Prognosis and survival in resected lung carcinoma based on the new international staging system. J Thorac Cardiovasc Surg. 1988;96:440–447. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

