Synaptotagmin-2 controls regulated exocytosis but not other secretory responses of mast cells
- PMID: 19473977
- PMCID: PMC2740570
- DOI: 10.1074/jbc.M109.002550
Synaptotagmin-2 controls regulated exocytosis but not other secretory responses of mast cells
Abstract
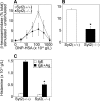
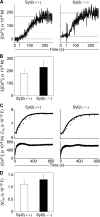
Mast cell degranulation is a highly regulated, calcium-dependent process, which is important for the acute release of inflammatory mediators during the course of many pathological conditions. We previously found that Synaptotagmin-2, a calcium sensor in neuronal exocytosis, was expressed in a mast cell line. We postulated that this protein may be involved in the control of mast cell-regulated exocytosis, and we generated Synaptotagmin-2 knock-out mice to test our hypothesis. Mast cells from this mutant animal conferred an abnormally decreased passive cutaneous anaphylaxis reaction on mast cell-deficient mice that correlated with a specific defect in mast cell-regulated exocytosis, leaving constitutive exocytosis and nonexocytic mast cell effector responses intact. This defect was not secondary to abnormalities in the development, maturation, migration, morphology, synthesis, and storage of inflammatory mediators, or intracellular calcium transients of the mast cells. Unlike neurons, the lack of Synaptotagmin-2 in mast cells was not associated with increased spontaneous exocytosis.
Figures




Similar articles
-
Impaired mast cell maturation and degranulation and attenuated allergic responses in Ndrg1-deficient mice.J Immunol. 2007 Jun 1;178(11):7042-53. doi: 10.4049/jimmunol.178.11.7042. J Immunol. 2007. PMID: 17513753
-
Munc18-2, but not Munc18-1 or Munc18-3, controls compound and single-vesicle-regulated exocytosis in mast cells.J Biol Chem. 2018 May 11;293(19):7148-7159. doi: 10.1074/jbc.RA118.002455. Epub 2018 Mar 29. J Biol Chem. 2018. PMID: 29599294 Free PMC article.
-
Effects of synaptotagmin 2 on membrane fusion between liposomes that contain SNAREs involved in exocytosis in mast cells.Biochim Biophys Acta. 2011 Oct;1808(10):2435-9. doi: 10.1016/j.bbamem.2011.07.003. Epub 2011 Jul 20. Biochim Biophys Acta. 2011. PMID: 21787744
-
Synaptotagmin regulates mast cell functions.Immunol Rev. 2001 Feb;179:25-34. doi: 10.1034/j.1600-065x.2001.790103.x. Immunol Rev. 2001. PMID: 11292024 Review.
-
Anaphylactic Degranulation of Mast Cells: Focus on Compound Exocytosis.J Immunol Res. 2019 Mar 18;2019:9542656. doi: 10.1155/2019/9542656. eCollection 2019. J Immunol Res. 2019. PMID: 31011586 Free PMC article. Review.
Cited by
-
Cytotoxic Granule Trafficking and Fusion in Synaptotagmin7-Deficient Cytotoxic T Lymphocytes.Front Immunol. 2020 May 29;11:1080. doi: 10.3389/fimmu.2020.01080. eCollection 2020. Front Immunol. 2020. PMID: 32547563 Free PMC article.
-
Structural requirements for the inhibition of calcium mobilization and mast cell activation by the pyrazole derivative BTP2.Int J Biochem Cell Biol. 2011 Aug;43(8):1228-39. doi: 10.1016/j.biocel.2011.04.016. Epub 2011 May 4. Int J Biochem Cell Biol. 2011. PMID: 21558014 Free PMC article.
-
Multiple roles for the actin cytoskeleton during regulated exocytosis.Cell Mol Life Sci. 2013 Jun;70(12):2099-121. doi: 10.1007/s00018-012-1156-5. Epub 2012 Sep 18. Cell Mol Life Sci. 2013. PMID: 22986507 Free PMC article. Review.
-
SNAP23 is essential for platelet and mast cell development and required in connective tissue mast cells for anaphylaxis.J Biol Chem. 2021 Jan-Jun;296:100268. doi: 10.1016/j.jbc.2021.100268. Epub 2021 Jan 8. J Biol Chem. 2021. PMID: 33837726 Free PMC article.
-
Munc13-4 reconstitutes calcium-dependent SNARE-mediated membrane fusion.J Cell Biol. 2012 Apr 16;197(2):301-12. doi: 10.1083/jcb.201109132. J Cell Biol. 2012. PMID: 22508512 Free PMC article.
References
-
- McNeil H. P., Shin K., Campbell I. K., Wicks I. P., Adachi R., Lee D. M., Stevens R. L. (2008) Arthritis Rheum. 58, 2338–2346 - PubMed
-
- Lee D. M., Friend D. S., Gurish M. F., Benoist C., Mathis D., Brenner M. B. (2002) Science 297, 1689–1692 - PubMed
-
- Thakurdas S. M., Melicoff E., Sansores-Garcia L., Moreira D. C., Petrova Y., Stevens R. L., Adachi R. (2007) J. Biol. Chem. 282, 20809–20815 - PubMed
-
- Malaviya R., Ikeda T., Ross E., Abraham S. N. (1996) Nature 381, 77–80 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

