Bovine and mouse SLO3 K+ channels: evolutionary divergence points to an RCK1 region of critical function
- PMID: 19473978
- PMCID: PMC2755883
- DOI: 10.1074/jbc.M109.015040
Bovine and mouse SLO3 K+ channels: evolutionary divergence points to an RCK1 region of critical function
Abstract
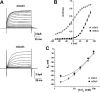
The slo3 gene encodes a K(+) channel found only in mammalian testis. This is in contrast to slo1, which is expressed in many tissues. Genes pertaining to male reproduction, especially those involved in sperm production, evolve morphologically and functionally much faster than their nonsexual counterparts. A comparison of SLO3 channel amino acid sequences from several species revealed a high degree of structural divergence relative to their SLO1 channel paralogues. To reveal any biophysical differences accompanying this rapid structural divergence, we analyzed the functional properties of SLO3 channels from two species, bovine and mouse. We observed several functional differences including voltage range of activation, kinetics, and pH sensitivity. Although SLO3 channel proteins from these two species lack conservation in many structural regions, we found that the first two of these three functional differences map to the same loop structure in their RCK1 (regulator of K(+) conductance 1) domain, which links the intermediate RCK1 subdomain to the C-terminal subdomain. We found that small structural changes in this region produce major changes in the voltage range of activation and kinetics. This rapidly evolving loop peptide shows the greatest length and sequence polymorphisms within RCK1 domains from many different species. In SLO3 channels this region may permit evolutionary changes that tune the gating properties in different species.
Figures







References
-
- Wyckoff G. J., Wang W., Wu C. I. (2000) Nature 403, 304–309 - PubMed
-
- Swanson W. J., Vacquier V. D. (2002) Nat. Rev. Genet. 3, 137–144 - PubMed
-
- Torgerson D. G., Kulathinal R. J., Singh R. S. (2002) Mol. Biol. Evol. 19, 1973–1980 - PubMed
-
- Schreiber M., Wei A., Yuan A., Gaut J., Saito M., Salkoff L. (1998) J. Biol. Chem. 273, 3509–3516 - PubMed
-
- Butler A., Tsunoda S., McCobb D. P., Wei A., Salkoff L. (1993) Science 261, 221–224 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

