STIM and Orai: dynamic intermembrane coupling to control cellular calcium signals
- PMID: 19473984
- PMCID: PMC2755655
- DOI: 10.1074/jbc.R109.018655
STIM and Orai: dynamic intermembrane coupling to control cellular calcium signals
Abstract
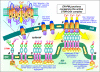
Ca(2+) signals controlling a vast array of cell functions involve both Ca(2+) store release and external Ca(2+) entry. These two events are coordinated through a dynamic intermembrane coupling between two distinct membrane proteins, STIM and Orai. STIM proteins are endoplasmic reticulum (ER) luminal Ca(2+) sensors that undergo a profound redistribution into discrete junctional ER domains closely juxtaposed with the plasma membrane (PM). Orai proteins are PM Ca(2+) channels that migrate and become tethered by STIM within the ER-PM junctions, where they mediate exceedingly selective Ca(2+) entry. We describe a new understanding of the nature of the proteins and how they function to mediate this remarkable intermembrane signaling process controlling Ca(2+) signals.
Figures

References
-
- Berridge M. J., Bootman M. D., Roderick H. L. (2003) Nat. Rev. Mol. Cell Biol. 4,517–529 - PubMed
-
- Parekh A. B., Penner R. (1997) Physiol. Rev. 77,901–930 - PubMed
-
- Venkatachalam K., van Rossum D. B., Patterson R. L., Ma H. T., Gill D. L. (2002) Nat. Cell Biol. 4,E263–E272 - PubMed
-
- Parekh A. B., Putney J. W., Jr. (2005) Physiol. Rev. 85,757–810 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

