Transcriptional activation by MEIS1A in response to protein kinase A signaling requires the transducers of regulated CREB family of CREB co-activators
- PMID: 19473990
- PMCID: PMC2707216
- DOI: 10.1074/jbc.M109.005090
Transcriptional activation by MEIS1A in response to protein kinase A signaling requires the transducers of regulated CREB family of CREB co-activators
Abstract
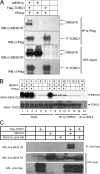
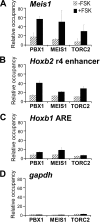
The transcription factor encoded by the murine ecotropic integration site 1 gene (MEIS1) is a partner of HOX and PBX proteins. It has been implicated in embryonic patterning and leukemia, and causally linked to restless legs syndrome. The MEIS1A C terminus harbors a transcriptional activation domain that is stimulated by protein kinase A (PKA) in a manner dependent on the co-activator of cAMP response element-binding protein (CREB), CREB-binding protein (CBP). We explored the involvement of another mediator of PKA-inducible transcription, namely the CREB co-activators transducers of regulated CREB activity (TORCs). Overexpression of TORC1 or TORC2 bypassed PKA for activation by MEIS1A. Co-immunoprecipitation experiments demonstrated a physical interaction between MEIS1 and TORC2 that is dependent on the MEIS1A C terminus, whereas chromatin immunoprecipitation revealed PKA-inducible recruitment of MEIS1, PBX1, and TORC2 on the MEIS1 target genes Hoxb2 and Meis1. The MEIS1 interaction domain on TORC1 was mapped to the N-terminal coiled-coil region, and TORC1 mutants lacking this domain attenuated the response to PKA on a natural MEIS1A target enhancer. Thus, TORCs physically cooperate with MEIS1 to achieve PKA-inducible transactivation through the MEIS1A C terminus, suggesting a concerted action in developmental and oncogenic processes.
Figures




Similar articles
-
MEIS C termini harbor transcriptional activation domains that respond to cell signaling.J Biol Chem. 2005 Mar 18;280(11):10119-27. doi: 10.1074/jbc.M413963200. Epub 2005 Jan 15. J Biol Chem. 2005. PMID: 15654074
-
A conserved motif N-terminal to the DNA-binding domains of myogenic bHLH transcription factors mediates cooperative DNA binding with pbx-Meis1/Prep1.Nucleic Acids Res. 1999 Sep 15;27(18):3752-61. doi: 10.1093/nar/27.18.3752. Nucleic Acids Res. 1999. PMID: 10471746 Free PMC article.
-
Persistent transactivation by meis1 replaces hox function in myeloid leukemogenesis models: evidence for co-occupancy of meis1-pbx and hox-pbx complexes on promoters of leukemia-associated genes.Mol Cell Biol. 2006 May;26(10):3902-16. doi: 10.1128/MCB.26.10.3902-3916.2006. Mol Cell Biol. 2006. PMID: 16648484 Free PMC article.
-
Coupling cAMP signaling to transcription in the liver: pivotal role of CREB and CREM.Exp Cell Res. 2002 May 1;275(2):143-54. doi: 10.1006/excr.2002.5491. Exp Cell Res. 2002. PMID: 11969286 Review.
-
Regulation of somatostatin gene transcription by cyclic adenosine monophosphate.Metabolism. 1996 Aug;45(8 Suppl 1):4-7. doi: 10.1016/s0026-0495(96)90068-2. Metabolism. 1996. PMID: 8769368 Review.
Cited by
-
Dual Role of CREB in The Regulation of VSMC Proliferation: Mode of Activation Determines Pro- or Anti-Mitogenic Function.Sci Rep. 2018 Mar 20;8(1):4904. doi: 10.1038/s41598-018-23199-4. Sci Rep. 2018. PMID: 29559698 Free PMC article.
-
Salt-inducible kinase 1-CREB-regulated transcription coactivator 1 signalling in the paraventricular nucleus of the hypothalamus plays a role in depression by regulating the hypothalamic-pituitary-adrenal axis.Mol Psychiatry. 2024 Jun;29(6):1660-1670. doi: 10.1038/s41380-022-01881-4. Epub 2022 Nov 25. Mol Psychiatry. 2024. PMID: 36434056
-
Dual actions of Meis1 inhibit erythroid progenitor development and sustain general hematopoietic cell proliferation.Blood. 2012 Jul 12;120(2):335-46. doi: 10.1182/blood-2012-01-403139. Epub 2012 Jun 4. Blood. 2012. PMID: 22665933 Free PMC article.
-
MEIS1-mediated transactivation of synaptotagmin-like 1 promotes CXCL12/CXCR4 signaling and leukemogenesis.J Clin Invest. 2016 May 2;126(5):1664-78. doi: 10.1172/JCI81516. Epub 2016 Mar 28. J Clin Invest. 2016. PMID: 27018596 Free PMC article.
-
Involvement of transducer of regulated cAMP response element-binding protein activity on corticotropin releasing hormone transcription.Endocrinology. 2010 Mar;151(3):1109-18. doi: 10.1210/en.2009-0963. Epub 2010 Jan 15. Endocrinology. 2010. PMID: 20080871 Free PMC article.
References
-
- Gehring W. J., Qian Y. Q., Billeter M., Furukubo-Tokunaga K., Schier A. F., Resendez-Perez D., Affolter M., Otting G., Wüthrich K. (1994) Cell 78, 211–223 - PubMed
-
- Bertolino E., Reimund B., Wildt-Perinic D., Clerc R. G. (1995) J. Biol. Chem. 270, 31178–31188 - PubMed
-
- Featherstone M. (2003) in Murine Homeobox Gene Control of Embryonic Patterning and Organogenesis (Lufkin T. ed) pp. 1–42, Elsevier, Amsterdam
-
- Moens C. B., Selleri L. (2006) Dev. Biol. 291, 193–206 - PubMed
-
- Calvo K. R., Knoepfler P., McGrath S., Kamps M. P. (1999) Oncogene 18, 8033–8043 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

