Differential regulation of transforming growth factor beta signaling pathways by Notch in human endothelial cells
- PMID: 19473993
- PMCID: PMC2740571
- DOI: 10.1074/jbc.M109.011833
Differential regulation of transforming growth factor beta signaling pathways by Notch in human endothelial cells
Abstract
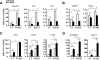
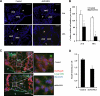
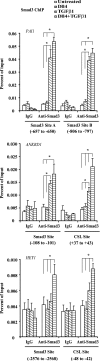
Notch and transforming growth factor beta (TGFbeta) play critical roles in endothelial-to-mesenchymal transition (EndMT), a process that is essential for heart development. Previously, we have shown that Notch and TGFbeta signaling synergistically induce Snail expression in endothelial cells, which is required for EndMT in cardiac cushion morphogenesis. Here, we report that Notch activation modulates TGFbeta signaling pathways in a receptor-activated Smad (R-Smad)-specific manner. Notch activation inhibits TGFbeta/Smad1 and TGFbeta/Smad2 signaling pathways by decreasing the expression of Smad1 and Smad2 and their target genes. In contrast, Notch increases SMAD3 mRNA expression and protein half-life and regulates the expression of TGFbeta/Smad3 target genes in a gene-specific manner. Inhibition of Notch in the cardiac cushion of mouse embryonic hearts reduces Smad3 expression. Notch and TGFbeta synergistically up-regulate a subset of genes by recruiting Smad3 to both Smad and CSL binding sites and cooperatively inducing histone H4 acetylation. This is the first evidence that Notch activation affects R-Smad expression and that cooperative induction of histone acetylation at specific promoters underlies the selective synergy between Notch and TGFbeta signaling pathways.
Figures





References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

