Deficiency of niemann-pick type C-1 protein impairs release of human immunodeficiency virus type 1 and results in Gag accumulation in late endosomal/lysosomal compartments
- PMID: 19474101
- PMCID: PMC2715784
- DOI: 10.1128/JVI.00259-09
Deficiency of niemann-pick type C-1 protein impairs release of human immunodeficiency virus type 1 and results in Gag accumulation in late endosomal/lysosomal compartments
Abstract
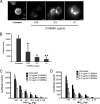
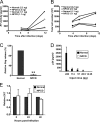
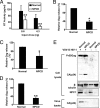
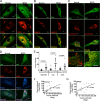
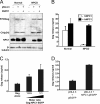
Human immunodeficiency virus type 1 (HIV-1) relies on cholesterol-laden lipid raft membrane microdomains for entry into and egress out of susceptible cells. In the present study, we examine the need for intracellular cholesterol trafficking pathways with respect to HIV-1 biogenesis using Niemann-Pick type C-1 (NPC1)-deficient (NPCD) cells, wherein these pathways are severely compromised, causing massive accumulation of cholesterol in late endosomal/lysosomal (LE/L) compartments. We have found that induction of an NPC disease-like phenotype through treatment of various cell types with the commonly used hydrophobic amine drug U18666A resulted in profound suppression of HIV-1 release. Further, NPCD Epstein-Barr virus-transformed B lymphocytes and fibroblasts from patients with NPC disease infected with a CD4-independent strain of HIV-1 or transfected with an HIV-1 proviral clone, respectively, replicated HIV-1 poorly compared to normal cells. Infection of the NPCD fibroblasts with a vesicular stomatitis virus G-pseudotyped strain of HIV-1 produced similar results, suggesting a postentry block to HIV-1 replication in these cells. Examination of these cells using confocal microscopy showed an accumulation and stabilization of Gag in LE/L compartments. Additionally, normal HIV-1 production could be restored in NPCD cells upon expression of a functional NPC1 protein, and overexpression of NPC1 increased HIV-1 release. Taken together, our findings demonstrate that intact intracellular cholesterol trafficking pathways mediated by NPC1 are needed for efficient HIV-1 production.
Figures

References
-
- Alroy, I., S. Tuvia, T. Greener, D. Gordon, H. M. Barr, D. Taglicht, R. Mandil-Levin, D. Ben-Avraham, D. Konforty, A. Nir, O. Levius, V. Bicoviski, M. Dori, S. Cohen, L. Yaar, O. Erez, O. Propheta-Meiran, M. Koskas, E. Caspi-Bachar, I. Alchanati, A. Sela-Brown, H. Moskowitz, U. Tessmer, U. Schubert, and Y. Reiss. 2005. The trans-Golgi network-associated human ubiquitin-protein ligase POSH is essential for HIV type 1 production. Proc. Natl. Acad. Sci. USA 1021478-1483. - PMC - PubMed
-
- Berger, A. C., G. Salazar, M. L. Styers, K. A. Newell-Litwa, E. Werner, R. A. Maue, A. H. Corbett, and V. Faundez. 2007. The subcellular localization of the Niemann-Pick Type C proteins depends on the adaptor complex AP-3. J. Cell Sci. 1203640-3652. - PubMed
-
- Bishop, N. E. 2003. Dynamics of endosomal sorting. Int. Rev. Cytol. 2321-57. - PubMed
-
- Campbell, S. M., S. M. Crowe, and J. Mak. 2001. Lipid rafts and HIV-1: from viral entry to assembly of progeny virions. J. Clin. Virol. 22217-227. - PubMed
-
- Carroll, K. S., J. Hanna, I. Simon, J. Krise, P. Barbero, and S. R. Pfeffer. 2001. Role of Rab9 GTPase in facilitating receptor recruitment by TIP47. Science 2921373-1376. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- U54 CA091408/CA/NCI NIH HHS/United States
- G12 RR003032/RR/NCRR NIH HHS/United States
- U54RR019192-04/RR/NCRR NIH HHS/United States
- U54 NS041071/NS/NINDS NIH HHS/United States
- U54CA91408/CA/NCI NIH HHS/United States
- U54 RR019192/RR/NCRR NIH HHS/United States
- R21 AI062408/AI/NIAID NIH HHS/United States
- U54NS041071-06/NS/NINDS NIH HHS/United States
- R01 HD040772/HD/NICHD NIH HHS/United States
- R01HD040772/HD/NICHD NIH HHS/United States
- G12RR03032-19/RR/NCRR NIH HHS/United States
- R21AI062408/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

