Baculovirus induces type I interferon production through toll-like receptor-dependent and -independent pathways in a cell-type-specific manner
- PMID: 19474102
- PMCID: PMC2708616
- DOI: 10.1128/JVI.00679-09
Baculovirus induces type I interferon production through toll-like receptor-dependent and -independent pathways in a cell-type-specific manner
Abstract
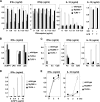
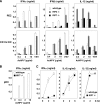
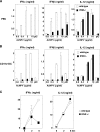
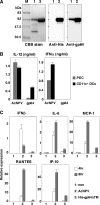
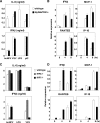
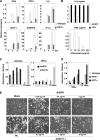
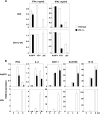
Autographa californica nuclear polyhedrosis virus (AcNPV) is a double-stranded-DNA virus that is pathogenic to insects. AcNPV was shown to induce an innate immune response in mammalian immune cells and to confer protection of mice from lethal viral infection. In this study, we have shown that production of type I interferon (IFN) by AcNPV in murine plasmacytoid dendritic cells (pDCs) and non-pDCs, such as peritoneal macrophages and splenic CD11c+ DCs, was mediated by Toll-like receptor (TLR)-dependent and -independent pathways, respectively. IFN regulatory factor 7 (IRF7) was shown to play a crucial role in the production of type I IFN by AcNPV not only in immune cells in vitro but also in vivo. In mouse embryonic fibroblasts (MEFs), AcNPV produced IFN-beta and IFN-inducible chemokines through TLR-independent and IRF3-dependent pathways, in contrast to the TLR-dependent and IRF3/IRF7-independent production of proinflammatory cytokines. Although production of IFN-beta and IFN-inducible chemokines was severely impaired in IFN promoter-stimulator 1 (IPS-1)-deficient MEFs upon infection with vesicular stomatitis virus, AcNPV produced substantial amounts of the cytokines in IPS-1-deficient MEFs. These results suggest that a novel signaling pathway(s) other than TLR- and IPS-1-dependent pathways participates in the production of type I IFN in response to AcNPV infection.
Figures
Similar articles
-
Involvement of the Toll-like receptor 9 signaling pathway in the induction of innate immunity by baculovirus.J Virol. 2005 Mar;79(5):2847-58. doi: 10.1128/JVI.79.5.2847-2858.2005. J Virol. 2005. PMID: 15709004 Free PMC article.
-
Innate immune response induced by baculovirus attenuates transgene expression in mammalian cells.J Virol. 2014 Feb;88(4):2157-67. doi: 10.1128/JVI.03055-13. Epub 2013 Dec 11. J Virol. 2014. PMID: 24335288 Free PMC article.
-
Conventional but not plasmacytoid dendritic cells foster the systemic virus-induced type I IFN response needed for efficient CD8 T cell priming.J Immunol. 2014 Aug 1;193(3):1151-61. doi: 10.4049/jimmunol.1301440. Epub 2014 Jun 27. J Immunol. 2014. PMID: 24973449 Free PMC article.
-
[Analysis of the application of host innate immune response to control and prevent infection].Uirusu. 2012 Jun;62(1):103-12. doi: 10.2222/jsv.62.103. Uirusu. 2012. PMID: 23189830 Review. Japanese.
-
[Toll-like receptors that sense viral infection].Uirusu. 2004 Jun;54(1):1-8. doi: 10.2222/jsv.54.1. Uirusu. 2004. PMID: 15449898 Review. Japanese.
Cited by
-
Baculovirus-mediated gene delivery and RNAi applications.Viruses. 2015 Apr 22;7(4):2099-125. doi: 10.3390/v7042099. Viruses. 2015. PMID: 25912715 Free PMC article. Review.
-
Immune responses induced by a combined vaccination with a recombinant chimera of Mycoplasma hyopneumoniae antigens and capsid virus-like particles of porcine circovirus type 2.BMC Vet Res. 2020 Sep 16;16(1):342. doi: 10.1186/s12917-020-02560-8. BMC Vet Res. 2020. PMID: 32938456 Free PMC article.
-
Baculovirus Vectors Induce the Production of Interferons in Swine: Their Potential in the Development of Antiviral Strategies.Vet Sci. 2021 Nov 17;8(11):278. doi: 10.3390/vetsci8110278. Vet Sci. 2021. PMID: 34822651 Free PMC article.
-
Baculovirus directly activates murine NK cells via TLR9.Cancer Gene Ther. 2017 Apr;24(4):175-179. doi: 10.1038/cgt.2017.2. Epub 2017 Feb 10. Cancer Gene Ther. 2017. PMID: 28186087
-
Solutions against emerging infectious and noninfectious human diseases through the application of baculovirus technologies.Appl Microbiol Biotechnol. 2021 Nov;105(21-22):8195-8226. doi: 10.1007/s00253-021-11615-1. Epub 2021 Oct 7. Appl Microbiol Biotechnol. 2021. PMID: 34618205 Free PMC article. Review.
References
-
- Abe, T., H. Takahashi, H. Hamazaki, N. Miyano-Kurosaki, Y. Matsuura, and H. Takaku. 2003. Baculovirus induces an innate immune response and confers protection from lethal influenza virus infection in mice. J. Immunol. 1711133-1139. - PubMed
-
- Bjorck, P. 2001. Isolation and characterization of plasmacytoid dendritic cells from Flt3 ligand and granulocyte-macrophage colony-stimulating factor-treated mice. Blood 983520-3526. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

