S6 kinase 1 knockout inhibits uninephrectomy- or diabetes-induced renal hypertrophy
- PMID: 19474189
- PMCID: PMC2739710
- DOI: 10.1152/ajprenal.00186.2009
S6 kinase 1 knockout inhibits uninephrectomy- or diabetes-induced renal hypertrophy
Abstract
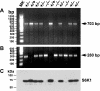
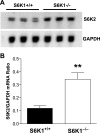
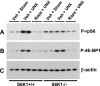
Removal of one kidney stimulates synthesis of RNA and protein, with minimal DNA replication, in all nephron segments of the remaining kidney, resulting in cell growth (increase in cell size) with minimal cell proliferation (increase in cell number). In addition to the compensatory renal hypertrophy caused by nephron loss, pathophysiological renal hypertrophy can occur as a consequence of early uncontrolled diabetes. However, the molecular mechanism underlying renal hypertrophy in these conditions remains unclear. In the present study, we report that deletion of S6 kinase 1 (S6K1) inhibited renal hypertrophy seen following either contralateral nephrectomy or induction of diabetes. In wild-type mice, hypertrophic stimuli increased phosphorylation of 40S ribosomal protein S6 (rpS6), a known target of S6K1. Immunoblotting analysis revealed that S6K1(-/-) mice exhibited moderately elevated basal levels of rpS6, which did not increase further in response to the hypertrophic stimuli. Northern blotting indicated a moderate upregulation of S6K2 expression in the kidneys of S6K1(-/-) mice. Phosphorylation of the eukaryotic translation initiation factor 4E-binding protein 1, another downstream target of the mammalian target of rapamycin (mTOR), was stimulated to equivalent levels in S6K1(-/-) and S6K1(+/+) littermates during renal hypertrophy, indicating that mTOR was still activated in the S6K1(-/-) mice. The highly selective mTOR inhibitor, rapamycin, inhibited increased phosphorylation of rpS6 and blocked 60-70% of the hypertrophy seen in wild-type mice but failed to prevent the approximately 10% hypertrophy seen in S6K1(-/-) mice in response to uninephrectomy (UNX) although it did inhibit the basal rpS6 phosphorylation. Thus the present study provides the first genetic evidence that S6K1 plays a major role in the development of compensatory renal hypertrophy as well as diabetic renal hypertrophy and indicates that UNX- and diabetes-mediated mTOR activation can selectively activate S6K1 without activating S6K2.
Figures





Comment in
-
The growing importance of mTORC1-S6K1 signaling in kidney.Am J Physiol Renal Physiol. 2009 Sep;297(3):F583-4. doi: 10.1152/ajprenal.00357.2009. Epub 2009 Jul 1. Am J Physiol Renal Physiol. 2009. PMID: 19570879 No abstract available.
Similar articles
-
Phosphorylation of ribosomal protein S6 mediates compensatory renal hypertrophy.Kidney Int. 2015 Mar;87(3):543-56. doi: 10.1038/ki.2014.302. Epub 2014 Sep 17. Kidney Int. 2015. PMID: 25229342 Free PMC article.
-
The growing importance of mTORC1-S6K1 signaling in kidney.Am J Physiol Renal Physiol. 2009 Sep;297(3):F583-4. doi: 10.1152/ajprenal.00357.2009. Epub 2009 Jul 1. Am J Physiol Renal Physiol. 2009. PMID: 19570879 No abstract available.
-
Role of mammalian target of rapamycin signaling in compensatory renal hypertrophy.J Am Soc Nephrol. 2005 May;16(5):1384-91. doi: 10.1681/ASN.2004100894. Epub 2005 Mar 23. J Am Soc Nephrol. 2005. PMID: 15788477
-
Regulation and function of ribosomal protein S6 kinase (S6K) within mTOR signalling networks.Biochem J. 2012 Jan 1;441(1):1-21. doi: 10.1042/BJ20110892. Biochem J. 2012. PMID: 22168436 Review.
-
Functions and regulation of the 70kDa ribosomal S6 kinases.Int J Biochem Cell Biol. 2011 Jan;43(1):47-59. doi: 10.1016/j.biocel.2010.09.018. Epub 2010 Oct 12. Int J Biochem Cell Biol. 2011. PMID: 20932932 Review.
Cited by
-
Impact of Cyclin B2 and Cell division cycle 2 on tubular hyperplasia in progressive chronic renal failure rats.Am J Physiol Renal Physiol. 2010 Apr;298(4):F923-34. doi: 10.1152/ajprenal.00567.2009. Epub 2010 Jan 13. Am J Physiol Renal Physiol. 2010. PMID: 20071461 Free PMC article.
-
Role of nutrient-sensing signals in the pathogenesis of diabetic nephropathy.Biomed Res Int. 2014;2014:315494. doi: 10.1155/2014/315494. Epub 2014 Jul 14. Biomed Res Int. 2014. PMID: 25126552 Free PMC article. Review.
-
Expression of leptin receptor in renal tubules is sparse but implicated in leptin-dependent kidney gene expression and function.Am J Physiol Renal Physiol. 2023 Jun 1;324(6):F544-F557. doi: 10.1152/ajprenal.00279.2022. Epub 2023 Apr 27. Am J Physiol Renal Physiol. 2023. PMID: 37102688 Free PMC article.
-
Roles of mTOR complexes in the kidney: implications for renal disease and transplantation.Nat Rev Nephrol. 2016 Oct;12(10):587-609. doi: 10.1038/nrneph.2016.108. Epub 2016 Aug 1. Nat Rev Nephrol. 2016. PMID: 27477490 Free PMC article. Review.
-
HIV-associated nephropathy: role of mammalian target of rapamycin pathway.Am J Pathol. 2010 Aug;177(2):813-21. doi: 10.2353/ajpath.2010.100131. Epub 2010 Jun 25. Am J Pathol. 2010. PMID: 20581056 Free PMC article.
References
-
- Abraham RT, Eng CH. Mammalian target of rapamycin as a therapeutic target in oncology. Expert Opin Ther Targets 12: 209–222, 2008. - PubMed
-
- Brown EJ, Beal PA, Keith CT, Chen J, Shin TB, Schreiber SL. Control of p70 s6 kinase by kinase activity of FRAP in vivo. Nature 377: 441–446, 1995. - PubMed
-
- Brunn GJ, Hudson CC, Sekulic A, Williams JM, Hosoi H, Houghton PJ, Lawrence JC Jr, Abraham RT. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science 277: 99–101, 1997. - PubMed
-
- Caramori ML, Mauer M. Diabetes and nephropathy. Curr Opin Nephrol Hypertens 12: 273–282, 2003. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

