Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice
- PMID: 19474305
- PMCID: PMC6665579
- DOI: 10.1523/JNEUROSCI.0887-09.2009
Human APOE isoform-dependent effects on brain beta-amyloid levels in PDAPP transgenic mice
Abstract
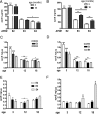
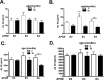
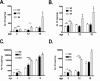
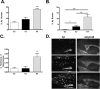
To investigate the role of human apolipoprotein E (apoE) on Abeta deposition in vivo, we crossed PDAPP mice lacking mouse Apoe to targeted replacement mice expressing human apoE (PDAPP/TRE2, PDAPP/TRE3, or PDAPP/TRE4). We then measured the levels of apoE protein and Abeta peptides in plasma, CSF, and brain homogenates in these mice at different ages. We also quantified the amount of brain Abeta and amyloid burden in 18-month-old mice. In young PDAPP/TRE4 mice that were analyzed at an age before brain Abeta deposition, we observed a significant decrease in the levels of apoE in CSF and brain when compared with age-matched mice expressing either human E2 or E3. The brain levels of Abeta42 in PDAPP/TRE4 mice were substantially elevated even at this very early time point. In older PDAPP/TRE4 mice, the levels of insoluble apoE protein increased in parallel to the dramatic rise in brain Abeta burden, and the majority of apoE was associated with Abeta. In TRE4 only mice, we also observed a significant decrease in the level of apoE in brain homogenates. Since the relative level of apoE mRNA was equivalent in PDAPP/TRE and TRE only mice, it appears that post-translational mechanisms influence the levels of apoE protein in brain (E4 < E3 << E2), resulting in early and dramatic apoE isoform-dependent effects on brain Abeta levels (E4 >> E3 > E2) that increase with age. Therapeutic strategies aimed at increasing the soluble levels of apoE protein, regardless of isoform, may effectively prevent and (or) treat Alzheimer's disease.
Figures


References
-
- Bales KR, Verina T, Dodel RC, Du Y, Altstiel L, Bender M, Hyslop P, Johnstone EM, Little SP, Cummins DJ, Piccardo P, Ghetti B, Paul SM. Lack of apolipoprotein E dramatically reduces amyloid β-peptide deposition. Nat Genet. 1997;17:263–264. - PubMed
-
- Bales KR, Verina T, Cummins DJ, Du Y, Dodel RC, Saura J, Fishman CE, DeLong CA, Piccardo P, Petegnief V, Ghetti B, Paul SM. Apolipoprotein E is essential for amyloid deposition in the APP V717F transgenic mouse model of Alzheimer's disease. Proc Natl Acad Sci U S A. 1999;96:15233–15238. - PMC - PubMed
-
- Beffert U, Cohn JS, Petit-Turcotte C, Tremblay M, Aumont N, Ramassamy C, Davignon J, Poirier J. Apolipoprotein E and beta-amyloid levels in the hippocampus and frontal cortex of Alzheimer's disease subjects are disease-related and apolipoprotein E genotype dependent. Brain Res. 1999;843:87–94. - PubMed
-
- Boschert U, Merlo-Pich E, Higgins G, Roses AD, Catsicas S. Apolipoprotein E expression by neurons surviving excitotoxic stress. Neurobiol Dis. 1999;6:508–514. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
