Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination
- PMID: 19474313
- PMCID: PMC2757755
- DOI: 10.1523/JNEUROSCI.0232-09.2009
Akt signals through the mammalian target of rapamycin pathway to regulate CNS myelination
Abstract
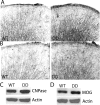
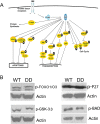
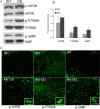
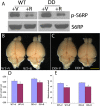
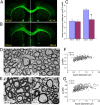
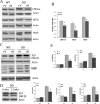
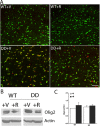
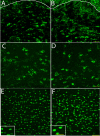
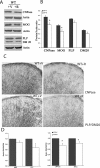
Mammalian target of rapamycin (mTOR), a well known Akt substrate, regulates multiple cellular functions including cell growth and protein synthesis. The current study identifies a novel role of the Akt/mTOR pathway as a regulator of CNS myelination. Previously, we showed that overexpressing constitutively active Akt in oligodendrocytes in a transgenic mouse model induces enhanced CNS myelination, with no changes in the proliferation or survival of oligodendrocyte progenitor or mature cells. The present study focused on the signaling mechanisms regulating this hypermyelination induced by Akt. Activation of mTOR and its downstream substrates (p70S6 kinase and S6 ribosomal protein) was observed in Akt-overexpressing oligodendrocytes. When mTOR signaling was inhibited chronically in vivo with rapamycin starting at 6 weeks of age, the observed hypermyelination was reduced to approximately the amount of myelin seen in wild-type mice. mTOR inhibition had little impact on wild-type myelination between 6 and 12 weeks of age, suggesting that, in normal adults, myelination is relatively complete and is no longer regulated by mTOR signaling. However, when mTOR was chronically inhibited in young adult wild-type mice, myelination was reduced. These results suggest that, during active myelination, the major Akt signal regulating CNS myelination is the mTOR pathway.
Figures
References
-
- Bhaskar PT, Hay N. The two TORCs and Akt. Dev Cell. 2007;12:487–502. - PubMed
-
- Brazil DP, Yang ZZ, Hemmings BA. Advances in protein kinase B signalling: AKTion on multiple fronts. Trends Biochem Sci. 2004;29:233–242. - PubMed
-
- Casaccia-Bonnefil P, Hardy RJ, Teng KK, Levine JM, Koff A, Chao MV. Loss of p27Kip1 function results in increased proliferative capacity of oligodendrocyte progenitors but unaltered timing of differentiation. Development. 1999;126:4027–4037. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous
