Pannexin 1: the molecular substrate of astrocyte "hemichannels"
- PMID: 19474335
- PMCID: PMC2733788
- DOI: 10.1523/JNEUROSCI.6062-08.2009
Pannexin 1: the molecular substrate of astrocyte "hemichannels"
Abstract
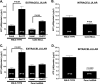
Purinergic signaling plays distinct and important roles in the CNS, including the transmission of calcium signals between astrocytes. Gap junction hemichannels are among the mechanisms proposed by which astrocytes might release ATP; however, whether the gap junction protein connexin43 (Cx43) forms these "hemichannels" remains controversial. Recently, a new group of proteins, the pannexins, have been shown to form nonselective, high-conductance plasmalemmal channels permeable to ATP, thereby offering an alternative for the hemichannel protein. Here, we provide strong evidence that, in cultured astrocytes, pannexin1 (Panx1) but not Cx43 forms hemichannels. Electrophysiological and fluorescence microscope recordings performed in wild-type and Cx43-null astrocytes did not reveal any differences in hemichannel activity, which was mostly eliminated by treating Cx43-null astrocytes with Panx1-short interfering RNA [Panx1-knockdown (Panx1-KD)]. Moreover, quantification of the amount of ATP released from wild-type, Cx43-null, and Panx1-KD astrocytes indicates that downregulation of Panx1, but not of Cx43, prevented ATP release from these cells.
Figures



References
-
- Bao L, Locovei S, Dahl G. Pannexin membrane channels are mechanosensitive conduits for ATP. FEBS Lett. 2004;572:65–68. - PubMed
-
- Bruzzone R, Barbe MT, Jakob NJ, Monyer H. Pharmacological properties of homomeric and heteromeric pannexin hemichannels expressed in Xenopus oocytes. J Neurochem. 2005;92:1033–1043. - PubMed
-
- Contreras JE, Sánchez HA, Eugenin EA, Speidel D, Theis M, Willecke K, Bukauskas FF, Bennett MV, Sáez JC. Metabolic inhibition induces opening of unapposed connexin 43 gap junction hemichannels and reduces gap junctional communication in cortical astrocytes in culture. Proc Natl Acad Sci U S A. 2002;99:495–500. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous
