High-affinity triplex targeting of double stranded DNA using chemically modified peptide nucleic acid oligomers
- PMID: 19474349
- PMCID: PMC2715256
- DOI: 10.1093/nar/gkp437
High-affinity triplex targeting of double stranded DNA using chemically modified peptide nucleic acid oligomers
Abstract
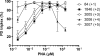
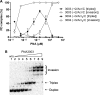
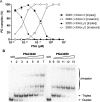
While sequence-selective dsDNA targeting by triplex forming oligonucleotides has been studied extensively, only very little is known about the properties of PNA-dsDNA triplexes--mainly due to the competing invasion process. Here we show that when appropriately modified using pseudoisocytosine substitution, in combination with (oligo)lysine or 9-aminoacridine conjugation, homopyrimidine PNA oligomers bind complementary dsDNA targets via triplex formation with (sub)nanomolar affinities (at pH 7.2, 150 mM Na(+)). Binding affinity can be modulated more than 1000-fold by changes in pH, PNA oligomer length, PNA net charge and/or by substitution of pseudoisocytosine for cytosine, and conjugation of the DNA intercalator 9-aminoacridine. Furthermore, 9-aminoacridine conjugation also strongly enhanced triplex invasion. Specificity for the fully matched target versus one containing single centrally located mismatches was more than 150-fold. Together the data support the use of homopyrimidine PNAs as efficient and sequence selective tools in triplex targeting strategies under physiological relevant conditions.
Figures


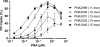



References
-
- Chin JY, Schleifman EB, Glazer PM. Repair and recombination induced by triple helix DNA. Front Biosci. 2007;12:4288–4297. - PubMed
-
- Rogers FA, Lloyd JA, Glazer PM. Triplex-forming oligonucleotides as potential tools for modulation of gene expression. Curr. Med. Chem. Anticancer Agents. 2005;5:319–326. - PubMed
-
- Dervan PB, Doss RM, Marques MA. Programmable DNA binding oligomers for control of transcription. Curr. Med. Chem. Anticancer Agents. 2005;5:373–387. - PubMed
-
- Porteus MH, Carroll D. Gene targeting using zinc finger nucleases. Nat. Biotechnol. 2005;23:967–973. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

