A muscle-targeting peptide displayed on AAV2 improves muscle tropism on systemic delivery
- PMID: 19474807
- PMCID: PMC2726895
- DOI: 10.1038/gt.2009.59
A muscle-targeting peptide displayed on AAV2 improves muscle tropism on systemic delivery
Abstract
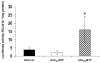
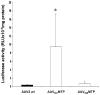
Adeno-associated virus (AAV) has become a leading gene transfer vector for striated muscles. However, the AAV vectors also exhibit broad tropisms after systemic delivery. In an attempt to improve muscle tropism, we inserted a 7-amino-acid (ASSLNIA) muscle-targeting peptide (MTP) in the capsids of AAV2 at residue 587 or 588, generating AAV(587)MTP and AAV(588)MTP. In vitro studies showed that both viruses diminished their infectivity on non-muscle cell lines as well as on un-differentiated myoblasts; however, preserved or enhanced their infectivity on differentiated myotubes. AAV(587)MTP, but not AAV(588)MTP, also abolished its heparin-binding capacity and infected myotubes in a heparin-independent manner. Furthermore, in vivo studies by intravenous vector administration in mice showed that AAV(587)MTP enhanced its tropism to various muscles and particularly to the heart (24.3-fold of unmodified AAV2), whereas reduced its tropism to the non-muscle tissues such as the liver, lungs, spleen and so on. This alteration of tissue tropism is not simply because of the loss of heparin-binding, as a mutant AAV2 (AAVHBSMut) containing heparin-binding site mutations lost infectivity on both non-muscle and muscle cells. Furthermore, free MTP peptide, but not the scrambled control peptide, competitively inhibited AAV(587)MTP infection on myotubes. These results suggest that AAV2 could be re-targeted to the striated muscles by a MTP inserted after residue 587 of the capsids. This proof of principle study showed first evidence of peptide-directed muscle targeting on systemic administration of AAV vectors.
Figures






Similar articles
-
Heparan Sulfate Binding Promotes Accumulation of Intravitreally Delivered Adeno-associated Viral Vectors at the Retina for Enhanced Transduction but Weakly Influences Tropism.J Virol. 2016 Oct 14;90(21):9878-9888. doi: 10.1128/JVI.01568-16. Print 2016 Nov 1. J Virol. 2016. PMID: 27558418 Free PMC article.
-
Insertional mutagenesis of the adeno-associated virus type 2 (AAV2) capsid gene and generation of AAV2 vectors targeted to alternative cell-surface receptors.Hum Gene Ther. 2001 Sep 20;12(14):1697-711. doi: 10.1089/104303401750476212. Hum Gene Ther. 2001. PMID: 11560765
-
Insertional mutagenesis at positions 520 and 584 of adeno-associated virus type 2 (AAV2) capsid gene and generation of AAV2 vectors with eliminated heparin- binding ability and introduced novel tropism.Hum Gene Ther. 2006 Mar;17(3):353-61. doi: 10.1089/hum.2006.17.353. Hum Gene Ther. 2006. PMID: 16544984
-
Designer gene delivery vectors: molecular engineering and evolution of adeno-associated viral vectors for enhanced gene transfer.Pharm Res. 2008 Mar;25(3):489-99. doi: 10.1007/s11095-007-9431-0. Epub 2007 Sep 1. Pharm Res. 2008. PMID: 17763830 Free PMC article. Review.
-
Adeno-associated viral vectors and their redirection to cell-type specific receptors.Adv Genet. 2009;67:29-60. doi: 10.1016/S0065-2660(09)67002-4. Adv Genet. 2009. PMID: 19914449 Review.
Cited by
-
Identification of Broadly Applicable Adeno-Associated Virus Vectors by Systematic Comparison of Commonly Used Capsid Variants In Vitro.Hum Gene Ther. 2022 Nov;33(21-22):1197-1212. doi: 10.1089/hum.2022.109. Epub 2022 Nov 2. Hum Gene Ther. 2022. PMID: 36097758 Free PMC article.
-
Polymers for improving the in vivo transduction efficiency of AAV2 vectors.PLoS One. 2010 Dec 28;5(12):e15576. doi: 10.1371/journal.pone.0015576. PLoS One. 2010. PMID: 21203395 Free PMC article.
-
Secretion of functional α1-antitrypsin is cell type dependent: Implications for intramuscular delivery for gene therapy.Proc Natl Acad Sci U S A. 2022 Aug 2;119(31):e2206103119. doi: 10.1073/pnas.2206103119. Epub 2022 Jul 28. Proc Natl Acad Sci U S A. 2022. PMID: 35901208 Free PMC article.
-
Combinatorial peptide libraries: mining for cell-binding peptides.Chem Rev. 2014 Jan 22;114(2):1020-81. doi: 10.1021/cr400166n. Epub 2013 Dec 3. Chem Rev. 2014. PMID: 24299061 Free PMC article. Review. No abstract available.
-
Delivery of recombinant adeno-associated virus vectors to rat diaphragm muscle via direct intramuscular injection.Hum Gene Ther Methods. 2013 Dec;24(6):364-71. doi: 10.1089/hgtb.2013.055. Epub 2013 Oct 11. Hum Gene Ther Methods. 2013. PMID: 24006956 Free PMC article.
References
-
- Cox GA, Cole NM, Matsumura K, Phelps SF, Hauschka SD, Campbell KP, et al. Overexpression of dystrophin in transgenic mdx mice eliminates dystrophic symptoms without toxicity. Nature. 1993;364:725–729. - PubMed
-
- Amalfitano A, McVie-Wylie AJ, Hu H, Dawson TL, Raben N, Plotz P, et al. Systemic correction of the muscle disorder glycogen storage disease type II after hepatic targeting of a modified adenovirus vector encoding human acid-alpha-glucosidase. Proc Natl Acad Sci U S A. 1999;96:8861–8866. - PMC - PubMed
-
- Greelish JP, Su LT, Lankford EB, Burkman JM, Chen H, Konig SK, et al. Stable restoration of the sarcoglycan complex in dystrophic muscle perfused with histamine and a recombinant adeno-associated viral vector. Nat Med. 1999;5:439–443. - PubMed
-
- Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, et al. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

