One-pot multicomponent coupling methods for the synthesis of diastereo- and enantioenriched (Z)-trisubstituted allylic alcohols
- PMID: 19476375
- PMCID: PMC2749706
- DOI: 10.1021/ja809821x
One-pot multicomponent coupling methods for the synthesis of diastereo- and enantioenriched (Z)-trisubstituted allylic alcohols
Abstract
(Z)-trisubstituted allylic alcohols are widespread structural motifs in natural products and biologically active compounds but are difficult to directly prepare. Introduced herein is a general one-pot multicomponent coupling method for the synthesis of (Z)-alpha,alpha,beta-trisubstituted allylic alcohols. (Z)-trisubstituted vinylzinc reagents are formed in situ by initial hydroboration of 1-bromo-1-alkynes. Addition of dialkylzinc reagents induces a 1,2-metalate rearrangement that is followed by a boron-to-zinc transmetalation. The resulting vinylzinc reagents add to a variety of prochiral aldehydes to produce racemic (Z)-trisubstituted allylic alcohols. When enantioenriched aldehyde substrates are employed, (Z)-trisubstituted allylic alcohols are isolated with high dr (>20:1 in many cases). For example, vinylation of enantioenriched benzyl-protected alpha- and beta-hydroxy propanal derivatives furnished the expected anti-Felkin addition products via chelation control. Surprisingly, silyl-protected alpha-hydroxy aldehydes also afford anti-Felkin addition products. A protocol for the catalytic asymmetric addition of (Z)-trisubstituted vinylzinc reagents to prochiral aldehydes with a (-)-MIB-based catalyst has also been developed. Several additives were investigated as inhibitors of the Lewis acidic alkylzinc halide byproducts, which promote the background reaction to form the racemate. Alpha-ethyl and alpha-cyclohexyl (Z)-trisubstituted allylic alcohols can now be synthesized with excellent levels of enantioselectivity in the presence of diamine inhibitors.
Figures






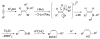

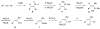


Similar articles
-
Catalytic asymmetric generation of (Z)-disubstituted allylic alcohols.J Am Chem Soc. 2007 Dec 26;129(51):16119-25. doi: 10.1021/ja0762285. Epub 2007 Dec 4. J Am Chem Soc. 2007. PMID: 18052173 Free PMC article.
-
Catalytic asymmetric reductive coupling of alkynes and aldehydes: enantioselective synthesis of allylic alcohols and alpha-hydroxy ketones.J Am Chem Soc. 2003 Mar 26;125(12):3442-3. doi: 10.1021/ja034366y. J Am Chem Soc. 2003. PMID: 12643701
-
A one-pot multicomponent coupling reaction for the stereocontrolled synthesis of (Z)-trisubstituted allylic alcohols.J Am Chem Soc. 2004 Mar 31;126(12):3702-3. doi: 10.1021/ja0396145. J Am Chem Soc. 2004. PMID: 15038709
-
Tandem reactions for streamlining synthesis: enantio- and diastereoselective one-pot generation of functionalized epoxy alcohols.Acc Chem Res. 2008 Aug;41(8):883-93. doi: 10.1021/ar800006h. Acc Chem Res. 2008. PMID: 18710197 Free PMC article. Review.
-
Asymmetric functional organozinc additions to aldehydes catalyzed by 1,1'-bi-2-naphthols (BINOLs).Acc Chem Res. 2014 May 20;47(5):1523-35. doi: 10.1021/ar500020k. Epub 2014 Apr 16. Acc Chem Res. 2014. PMID: 24738985 Free PMC article. Review.
Cited by
-
Stereoisomerically pure trisubstituted vinylaluminum reagents and their utility in copper-catalyzed enantioselective synthesis of 1,4-dienes containing Z or E alkenes.Angew Chem Int Ed Engl. 2010;49(2):419-23. doi: 10.1002/anie.200905223. Angew Chem Int Ed Engl. 2010. PMID: 19967689 Free PMC article. No abstract available.
-
Overriding Felkin control: a general method for highly diastereoselective chelation-controlled additions to alpha-silyloxy aldehydes.J Am Chem Soc. 2010 Mar 31;132(12):4399-408. doi: 10.1021/ja910717p. J Am Chem Soc. 2010. PMID: 20218659 Free PMC article.
-
Access to stereodefined (Z)-allylsilanes and (Z)-allylic alcohols via cobalt-catalyzed regioselective hydrosilylation of allenes.Nat Commun. 2017 Dec 22;8(1):2258. doi: 10.1038/s41467-017-02382-7. Nat Commun. 2017. PMID: 29273720 Free PMC article.
-
Highly diastereoselective chelation-controlled additions to α-silyloxy ketones.J Am Chem Soc. 2011 May 25;133(20):7969-76. doi: 10.1021/ja201629d. Epub 2011 May 2. J Am Chem Soc. 2011. PMID: 21534530 Free PMC article.
-
A general strategy for regiocontrol in nickel-catalyzed reductive couplings of aldehydes and alkynes.J Am Chem Soc. 2010 May 12;132(18):6304-5. doi: 10.1021/ja102262v. J Am Chem Soc. 2010. PMID: 20394367 Free PMC article.
References
-
- Walsh PJ, Kozlowski MC. Fundamentals of Asymmetric Catalysis. University Science Books; Sausalito: 2008.
-
- Pu L, Yu HB. Chem Rev. 2001;101:757–824. - PubMed
-
- Corey EJ, Helal CJ. Angew Chem Int Ed. 1998;37:1986–2012. - PubMed
-
- Ohkuma T, Koizumi M, Doucet H, Pham T, Kozawa M, Murata K, Katayama E, Yokozawa T, Ikariya T, Noyori R. J Am Chem Soc. 1998;120:13529–13530.
-
- Noyori R, Ohkuma T. Angew Chem Int Ed Engl. 2001;40:40–73. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

