Cellular calcium deficiency plays a role in neuronal death caused by proteasome inhibitors
- PMID: 19476541
- PMCID: PMC2690718
- DOI: 10.1111/j.1471-4159.2009.06037.x
Cellular calcium deficiency plays a role in neuronal death caused by proteasome inhibitors
Abstract
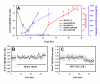
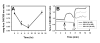
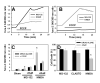
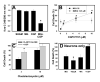
Cytosolic Ca(2+) concentration ([Ca(2+)](i)) is reduced in cultured neurons undergoing neuronal death caused by inhibitors of the ubiquitin proteasome system. Activation of calcium entry via voltage-gated Ca(2+) channels restores cytosolic Ca(2+) levels and reduces this neuronal death (Snider et al. 2002). We now show that this reduction in [Ca(2+)](i) is transient and occurs early in the cell death process, before activation of caspase 3. Agents that increase Ca(2+) influx such as activation of voltage-gated Ca(2+) channels or stimulation of Ca(2+) entry via the plasma membrane Na-Ca exchanger attenuate neuronal death only if applied early in the cell death process. Cultures treated with proteasome inhibitors had reduced current density for voltage-gated Ca(2+) channels and a less robust increase in [Ca(2+)](i) after depolarization. Levels of endoplasmic reticulum Ca(2+) were reduced and capacitative Ca(2+) entry was impaired early in the cell death process. Mitochondrial Ca(2+) was slightly increased. Preventing the transfer of Ca(2+) from mitochondria to cytosol increased neuronal vulnerability to this death while blockade of mitochondrial Ca(2+) uptake via the uniporter had no effect. Programmed cell death induced by proteasome inhibition may be caused in part by an early reduction in cytosolic and endoplasmic reticulum Ca(2+,) possibly mediated by dysfunction of voltage-gated Ca(2+) channels. These findings may have implications for the treatment of disorders associated with protein misfolding in which proteasome impairment and programmed cell death may occur.
Figures



References
-
- Canzoniero LM, Babcock DJ, Gottron FJ, et al. Raising intracellular calcium attenuates neuronal apoptosis triggered by staurosporine or oxygen-glucose deprivation in the presence of glutamate receptor blockade. Neurobiol. Dis. 2004;15:520–528. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

