Use of an electronic medical record to characterize cases of intermediate statin-induced muscle toxicity
- PMID: 19476582
- PMCID: PMC3773543
- DOI: 10.1111/j.1751-7141.2009.00028.x
Use of an electronic medical record to characterize cases of intermediate statin-induced muscle toxicity
Abstract
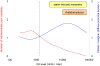
Statin use can be accompanied by a variety of musculoskeletal complaints. The authors describe the clinical characteristics of case patients who experienced adverse statin-induced musculoskeletal symptoms within a large population-based cohort in Central Wisconsin. Case status was determined based on elevated serum creatine kinase (CK) levels and the presence of at least 1 physician note reflecting an increased index of suspicion for statin intolerance. From the medical records of nearly 2 million unique patients, the authors identified more than 20,000 potential study patients ( approximately 1%) having CK data and at least 1 exposure to a statin drug. Manual screening was completed on 2227 patients with CK levels in the upper 10th percentile. Of those screened, 267 met inclusion criteria (12.0% eligibility) and 218 agreed to participate in a retrospective study characterizing the risk determinants of statin-induced muscle toxicity. Three categoric pain variables were graded retrospectively (distribution, location, and severity of pain). The presenting complaints of the case patients were extremely heterogeneous. The number of patients with a compelling pain syndrome (diffuse, proximal muscle pain of high intensity) increased at higher serum CK levels; the number of patients with indeterminate pain variables decreased at higher serum CK levels. The lines reflecting these relationships cross at a CK level of approximately 1175 U/L, approximately half the threshold level needed to make a clinical diagnosis of "myopathy" (ie, CK >10-fold the upper limit of normal).
(c) 2009 Wiley Periodicals, Inc.
Figures


References
-
- Thompson PD, Clarkson PM, Rosenson RS. An Assessment of Statin Safety by Muscle Experts. The American Journal of Cardiology. 2006;97(8, Supplement 1):S69–S76. - PubMed
-
- Wilke RA, Moore JH, Burmester JK. Relative impact of CYP3A genotype and concomitant medication on the severity of atorvastatin-induced muscle damage. Pharmacogenet Genomics. 2005;15(6):415–21. - PubMed
-
- Wilke RA, Reif DM, Moore JH. Combinatorial Pharmacogenetics. Nature Reviews Drug Discovery. 2005;4(11):911–8. - PubMed
-
- SEARCH Collaborative Group. Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R. SLCO1B1 variants and statin-induced myopathy-a genomewide study. N Engl J Med. 2008;359(8):789–99. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

