Improved surgical outcomes for breast cancer patients receiving neoadjuvant aromatase inhibitor therapy: results from a multicenter phase II trial
- PMID: 19476859
- PMCID: PMC3683862
- DOI: 10.1016/j.jamcollsurg.2009.01.035
Improved surgical outcomes for breast cancer patients receiving neoadjuvant aromatase inhibitor therapy: results from a multicenter phase II trial
Abstract
Background: Neoadjuvant aromatase inhibitor therapy has been reported to improve surgical outcomes for postmenopausal women with clinical stage II or III hormone receptor-positive breast cancer. A multicenter phase II clinical trial was conducted to investigate the value of this approach for US surgical practice.
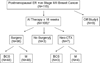
Study design: One hundred fifteen postmenopausal women with >2 cm, estrogen receptor (ER) or progesterone receptor (PgR)-positive breast cancer were enrolled in a trial of 16 to 24 weeks of letrozole 2.5 mg daily before operation.
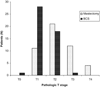
Results: One hundred six patients were eligible for primary analysis, 96 underwent operations, 7 received chemotherapy after progressive disease, and 3 did not undergo an operation. Baseline surgical status was marginal for breast-conserving surgery (BCS) in 48 (45%), 47 were definitely ineligible for BCS (44%), and 11 were inoperable by standard mastectomy (10%). Overall Response Evaluation Criteria In Solid Tumors clinical response rate in the breast was 62%, with 12% experiencing progressive disease. Fifty percent underwent BCS, including 30 of 46 (65%) patients who were initially marginal for BCS and 15 of 39 (38%) patients who were initially ineligible for BCS. All 11 inoperable patients successfully underwent operations, including 3 (27%) who had BCS. Nineteen percent of patients undergoing mastectomy had a pathologic T1 tumor, suggesting that some highly responsive tumors were overtreated surgically.
Conclusions: Neoadjuvant aromatase inhibitor improves operability and facilitates BCS, but there was considerable variability in responsiveness. Better techniques to predict response, determine residual tumor burden before operation, and greater willingness to attempt BCS in responsive patients could additionally improve the rate of successful BCS.
Figures

References
-
- Wolmark N, Wang J, Mamounas E, et al. Preoperative chemotherapy in patients with operable breast cancer: nine-year results from National Surgical Adjuvant Breast and Bowel Project B-18. J Natl Cancer Inst Monogr. 2001:96–102. - PubMed
-
- Bear HD, Anderson S, Smith RE, et al. Sequential preoperative or postoperative docetaxel added to preoperative doxorubicin plus cyclophosphamide for operable breast cancer: National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2006;24:2019–2027. - PubMed
-
- Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National Surgical Adjuvant Breast and Bowel Project Protocols B-18 and B-27. J Clin Oncol. 2008;26:778–785. - PubMed
-
- Barnes DM, Millis RR, Gillett CE, et al. The interaction of oestrogen receptor status and pathological features with adjuvant treatment in relation to survival in patients with operable breast cancer: a retrospective study of 2660 patients. Endocr Relat Cancer. 2004;11:85–96. - PubMed
-
- Ma CX, Ellis MJ. Neoadjuvant endocrine therapy for locally advanced breast cancer. Semin Oncol. 2006;33:650–656. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

