Relative importance of complement-mediated bactericidal and opsonic activity for protection against meningococcal disease
- PMID: 19477054
- PMCID: PMC2751589
- DOI: 10.1016/j.vaccine.2009.04.066
Relative importance of complement-mediated bactericidal and opsonic activity for protection against meningococcal disease
Abstract
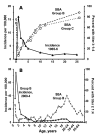
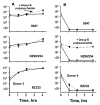
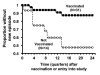
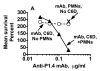
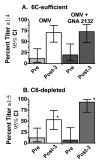
Killing of Neisseria meningitidis can result from complement-mediated serum bactericidal activity (SBA) or opsonophagocytosis (OPA), or a combination of the two mechanisms. While SBA titers > or =1:4 confer protection, recent evidence suggests that this threshold titer may not be required. For example, the incidence of meningococcal disease declines between ages 1 and 4 years without evidence of acquisition of SBA titers > or =1:4. Meningococcal polysaccharide vaccination also elicited OPA and lowered the risk of disease in patients with late complement component deficiencies whose sera did not support SBA. Sera from healthy adults immunized with an outer membrane vesicle vaccine showed OPA killing of N. meningitidis with C6-depleted complement, and whole blood from complement-sufficient non-immunized adults with SBA titers <1:4 also frequently had killing activity. Collectively the data indicate that SBA titers <1:4 and/or vaccine-induced OPA can confer protection against meningococcal disease.
Conflict of interest statement
Conflict of interest. The author is principal investigator of laboratory research conducted on behalf of Children's Hospital Oakland Research Institute that is funded by grants from Novartis Vaccines and Diagnostics, and Sanofi Pasteur. He also holds a paid consultancy from Novartis and is an inventor on patents or patent applications in the area of meningococcal B vaccines.
Figures
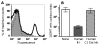

Similar articles
-
Bactericidal antibody is the immunologic surrogate of protection against meningococcal disease.Vaccine. 2009 Jun 24;27 Suppl 2:B112-6. doi: 10.1016/j.vaccine.2009.04.065. Epub 2009 May 21. Vaccine. 2009. PMID: 19464093 Review.
-
Meningococcal Serogroup A, B, C, W, X, and Y Serum Bactericidal Antibody Assays.Methods Mol Biol. 2019;1969:169-179. doi: 10.1007/978-1-4939-9202-7_12. Methods Mol Biol. 2019. PMID: 30877677
-
Ex vivo model of meningococcal bacteremia using human blood for measuring vaccine-induced serum passive protective activity.Clin Vaccine Immunol. 2009 Jun;16(6):785-91. doi: 10.1128/CVI.00007-09. Epub 2009 Apr 1. Clin Vaccine Immunol. 2009. PMID: 19339487 Free PMC article.
-
Naturally acquired passive protective activity against Neisseria meningitidis Group C in the absence of serum bactericidal activity.Infect Immun. 2004 Oct;72(10):5903-9. doi: 10.1128/IAI.72.10.5903-5909.2004. Infect Immun. 2004. PMID: 15385492 Free PMC article.
-
Serum bactericidal antibody assays - The role of complement in infection and immunity.Vaccine. 2015 Aug 26;33(36):4414-21. doi: 10.1016/j.vaccine.2015.07.019. Epub 2015 Jul 15. Vaccine. 2015. PMID: 26187262 Review.
Cited by
-
A broadly cross-reactive monoclonal antibody against an epitope on the n-terminus of meningococcal fHbp.Sci Rep. 2012;2:341. doi: 10.1038/srep00341. Epub 2012 Mar 28. Sci Rep. 2012. PMID: 22461972 Free PMC article.
-
Utilization of serologic assays to support efficacy of vaccines in nonclinical and clinical trials: meeting at the crossroads.Vaccine. 2010 Jun 23;28(29):4539-47. doi: 10.1016/j.vaccine.2010.04.094. Epub 2010 May 12. Vaccine. 2010. PMID: 20470795 Free PMC article.
-
A live attenuated vaccine to prevent severe neonatal Escherichia coli K1 infections.Nat Commun. 2024 Apr 8;15(1):3021. doi: 10.1038/s41467-024-46775-x. Nat Commun. 2024. PMID: 38589401 Free PMC article.
-
Proteomics, Bioinformatics and Structure-Function Antigen Mining For Gonorrhea Vaccines.Front Immunol. 2018 Dec 4;9:2793. doi: 10.3389/fimmu.2018.02793. eCollection 2018. Front Immunol. 2018. PMID: 30564232 Free PMC article. Review.
-
Complement and Bacterial Infections: From Molecular Mechanisms to Therapeutic Applications.J Innate Immun. 2018;10(5-6):455-464. doi: 10.1159/000491439. Epub 2018 Aug 27. J Innate Immun. 2018. PMID: 30149378 Free PMC article. Review.
References
-
- Granoff DM, Harrison L, Borrow R. Meningoccal Vaccines. In: Plotkin SA, Offit P, Orenstein WA, editors. Vaccines. 5th. Philadelphia: Saunders Elsevier; 2008. pp. 399–434.
-
- Schneider MC, Exley RM, Ram S, Sim RB, Tang CM. Interactions between Neisseria meningitidis and the complement system. Trends Microbiol. 2007;15(5):233–40. - PubMed
-
- Ram S, Volgel U. Role of complement in defense against meningococcal infections. In: Frosch M, Maiden MC, editors. Handbook of Meningococcal Disease: Meningococcal disease, pathogenicity and prevention. Weinheim, Germany: Wiley-VCV; 2006. pp. 273–93.
-
- Sprong T, Brandtzaeg P, Fung M, Pharo AM, Hoiby EA, Michaelsen TE, et al. Inhibition of C5a-induced inflammation with preserved C5b-9-mediated bactericidal activity in a human whole blood model of meningococcal sepsis. Blood. 2003;102(10):3702–10. - PubMed
-
- Söderström C, Braconier JH, Danielsson D, Sjöholm AG. Bactericidal activity for Neisseria meningitidis in properdin-deficient sera. J Infect Dis. 1987;156(1):107–12. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

