Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression
- PMID: 19477151
- PMCID: PMC2759281
- DOI: 10.1016/j.neuron.2009.04.017
Neurogenesis-dependent and -independent effects of fluoxetine in an animal model of anxiety/depression
Abstract
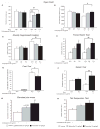
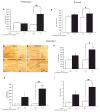
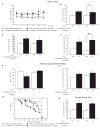
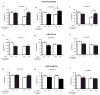
Understanding the physiopathology of affective disorders and their treatment relies on the availability of experimental models that accurately mimic aspects of the disease. Here we describe a mouse model of an anxiety/depressive-like state induced by chronic corticosterone treatment. Furthermore, chronic antidepressant treatment reversed the behavioral dysfunctions and the inhibition of hippocampal neurogenesis induced by corticosterone treatment. In corticosterone-treated mice where hippocampal neurogenesis is abolished by X-irradiation, the efficacy of fluoxetine is blocked in some, but not all, behavioral paradigms, suggesting both neurogenesis-dependent and -independent mechanisms of antidepressant action. Finally, we identified a number of candidate genes, the expression of which is decreased by chronic corticosterone and normalized by chronic fluoxetine treatment selectively in the hypothalamus. Importantly, mice deficient in one of these genes, beta-arrestin 2, displayed a reduced response to fluoxetine in multiple tasks, suggesting that beta-arrestin signaling is necessary for the antidepressant effects of fluoxetine.
Figures

Comment in
-
Dissecting the pathophysiology of depression with a Swiss army knife.Neuron. 2009 May 28;62(4):453-5. doi: 10.1016/j.neuron.2009.05.004. Neuron. 2009. PMID: 19477146
References
-
- Airan RD, Meltzer LA, Roy M, Gong Y, Chen H, Deisseroth K. High-speed imaging reveals neurophysiological links to behavior in an animal model of depression. Science. 2007;317:819–823. - PubMed
-
- Antonijevic IA. Depressive disorders - is it time to endorse different pathophysiologies? Psychoneuroendocrinology. 2006;31:1–15. - PubMed
-
- Ardayfio P, Kim KS. Anxiogenic-like effect of chronic corticosterone in the light-dark emergence task in mice. Behav Neurosci. 2006;120:249–256. - PubMed
-
- Artigas F, Romero L, de Montigny C, Blier P. Acceleration of the effect of selected antidepressant drugs in major depression by 5-HT1A antagonists. Trends Neurosci. 1996;19:378–383. - PubMed
-
- Avissar S, Matuzany-Ruban A, Tzukert K, Schreiber G. Beta-arrestin-1 levels: reduced in leukocytes of patients with depression and elevated by antidepressants in rat brain. Am J Psychiatry. 2004;161:2066–2072. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

