Inhibition of ATP synthase by chlorinated adenosine analogue
- PMID: 19477165
- PMCID: PMC2763632
- DOI: 10.1016/j.bcp.2009.05.019
Inhibition of ATP synthase by chlorinated adenosine analogue
Abstract
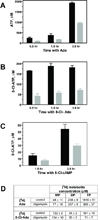
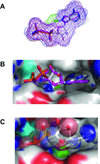
8-Chloroadenosine (8-Cl-Ado) is a ribonucleoside analogue that is currently in clinical trial for chronic lymphocytic leukemia. Based on the decline in cellular ATP pool following 8-Cl-Ado treatment, we hypothesized that 8-Cl-ADP and 8-Cl-ATP may interfere with ATP synthase, a key enzyme in ATP production. Mitochondrial ATP synthase is composed of two major parts; F(O) intermembrane base and F1 domain, containing alpha and beta subunits. Crystal structures of both alpha and beta subunits that bind to the substrate, ADP, are known in tight binding (alpha(dp)beta(dp)) and loose binding (alpha(tp)beta(tp)) states. Molecular docking demonstrated that 8-Cl-ADP/8-Cl-ATP occupied similar binding modes as ADP/ATP in the tight and loose binding sites of ATP synthase, respectively, suggesting that the chlorinated nucleotide metabolites may be functional substrates and inhibitors of the enzyme. The computational predictions were consistent with our whole cell biochemical results. Oligomycin, an established pharmacological inhibitor of ATP synthase, decreased both ATP and 8-Cl-ATP formation from exogenous substrates, however, did not affect pyrimidine nucleoside analogue triphosphate accumulation. Synthesis of ATP from ADP was inhibited in cells loaded with 8-Cl-ATP. These biochemical studies are in consent with the computational modeling; in the alpha(tp)beta(tp) state 8-Cl-ATP occupies similar binding as ANP, a non-hydrolyzable ATP mimic that is a known inhibitor. Similarly, in the substrate binding site (alpha(dp)beta(dp)) 8-Cl-ATP occupies a similar position as ATP mimic ADP-BeF(3)(-). Collectively, our current work suggests that 8-Cl-ADP may serve as a substrate and the 8-Cl-ATP may be an inhibitor of ATP synthase.
Figures




Similar articles
-
8-chloro-cAMP and 8-chloro-adenosine act by the same mechanism in multiple myeloma cells.Cancer Res. 2001 Jul 15;61(14):5474-9. Cancer Res. 2001. PMID: 11454694
-
Intracellular succinylation of 8-chloroadenosine and its effect on fumarate levels.J Biol Chem. 2010 Mar 12;285(11):8022-30. doi: 10.1074/jbc.M109.085803. Epub 2010 Jan 11. J Biol Chem. 2010. PMID: 20064937 Free PMC article.
-
Preclinical activity of 8-chloroadenosine with mantle cell lymphoma: roles of energy depletion and inhibition of DNA and RNA synthesis.Br J Haematol. 2009 Nov;147(3):297-307. doi: 10.1111/j.1365-2141.2009.07850.x. Epub 2009 Aug 25. Br J Haematol. 2009. PMID: 19709085 Free PMC article.
-
Catalytic site forms and controls in ATP synthase catalysis.Biochim Biophys Acta. 2000 May 31;1458(2-3):252-62. doi: 10.1016/s0005-2728(00)00077-3. Biochim Biophys Acta. 2000. PMID: 10838041 Review.
-
Understanding ATP synthesis: structure and mechanism of the F1-ATPase (Review).Mol Membr Biol. 2003 Jan-Mar;20(1):27-33. doi: 10.1080/0968768031000066532. Mol Membr Biol. 2003. PMID: 12745923 Review.
Cited by
-
E2F1-mediated DNA damage is implicated in 8-Cl-adenosine-induced chromosome missegregation and apoptosis in human lung cancer H1299 cells.Mol Cell Biochem. 2013 Dec;384(1-2):187-96. doi: 10.1007/s11010-013-1797-1. Epub 2013 Sep 15. Mol Cell Biochem. 2013. PMID: 24037421
-
8-Chloroadenosine induces apoptosis in human coronary artery endothelial cells through the activation of the unfolded protein response.Redox Biol. 2019 Sep;26:101274. doi: 10.1016/j.redox.2019.101274. Epub 2019 Jul 10. Redox Biol. 2019. PMID: 31307008 Free PMC article.
-
Ginsenoside Rh2 stimulates the production of mitochondrial reactive oxygen species and induces apoptosis of cervical cancer cells by inhibiting mitochondrial electron transfer chain complex.Mol Med Rep. 2021 Dec;24(6):873. doi: 10.3892/mmr.2021.12513. Epub 2021 Oct 29. Mol Med Rep. 2021. PMID: 34713297 Free PMC article.
-
Recent development of anticancer therapeutics targeting Akt.Recent Pat Anticancer Drug Discov. 2011 Jan;6(1):146-59. doi: 10.2174/157489211793980079. Recent Pat Anticancer Drug Discov. 2011. PMID: 21110830 Free PMC article. Review.
-
Integrative gene expression profiling reveals G6PD-mediated resistance to RNA-directed nucleoside analogues in B-cell neoplasms.PLoS One. 2012;7(7):e41455. doi: 10.1371/journal.pone.0041455. Epub 2012 Jul 27. PLoS One. 2012. PMID: 22848499 Free PMC article.
References
-
- Plunkett W, Gandhi V. Purine and pyrimidine nucleoside analogs. Cancer Chemother Biol Response Modif. 2001;19:21–45. - PubMed
-
- Bonate PL, Arthaud L, Cantrell WR, Jr, Stephenson K, Secrist JA, 3rd, Weitman S. Discovery and development of clofarabine: a nucleoside analogue for treating cancer. Nat Rev Drug Discov. 2006;5:855–863. - PubMed
-
- Ho AD, Hensel M. Pentostatin for the treatment of indolent lymphoproliferative disorders. Semin Hematol. 2006;43:S2–S10. - PubMed
-
- Kay NE. Purine analogue-based chemotherapy regimens for patients with previously untreated B-chronic lymphocytic leukemia. Semin Hematol. 2006;43:S50–S54. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

