Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles
- PMID: 19477208
- PMCID: PMC2746980
- DOI: 10.1016/j.jconrel.2009.05.026
Controlled and targeted tumor chemotherapy by ultrasound-activated nanoemulsions/microbubbles
Abstract
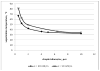
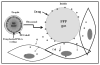
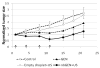
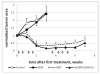
The paper reports the results of nanotherapy of ovarian, breast, and pancreatic cancerous tumors by paclitaxel-loaded nanoemulsions that convert into microbubbles locally in tumor tissue under the action of tumor-directed therapeutic ultrasound. Tumor accumulation of nanoemulsions was confirmed by ultrasound imaging. Dramatic regression of ovarian, breast, and orthotopic pancreatic tumors was observed in tumor therapy through systemic injections of drug-loaded nanoemulsions combined with therapeutic ultrasound, signifying efficient ultrasound-triggered drug release from tumor-accumulated nanodroplets. The mechanism of drug release in the process of droplet-to-bubble conversion is discussed. No therapeutic effect from the nanodroplet/ultrasound combination was observed without the drug, indicating that therapeutic effect was caused by the ultrasound-enhanced chemotherapeutic action of the tumor-targeted drug, rather than the mechanical or thermal action of ultrasound itself. Tumor recurrence was observed after the completion of the first treatment round; a second treatment round with the same regimen proved less effective, suggesting that drug-resistant cells were either developed or selected during the first treatment round.
Figures









References
-
- Campbell R. Tumor physiology and delivery of nanopharmaceuticals. Anticancer Agents Med Chem. 2006;6(6):503–512. - PubMed
-
- Iyer AK, Khaled G, Fang J, Maeda H. Exploiting the enhanced permeability and retention effect for tumor targeting. Drug Discov Today. 2006;11(17–18):812–818. - PubMed
-
- Roberts WG, Palade GE. Neovasculature induced by vascular endothelial factor is fenestrated. Cancer Res. 1997;57(4):765–772. - PubMed
-
- Rapoport N. Physical stimuli-responsive polymeric micelles for anti-cancer drug delivery. Progr Polymer Sci. 2007;32(8–9):962–990.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

