Chronic ethanol feeding potentiates Fas Jo2-induced hepatotoxicity: role of CYP2E1 and TNF-alpha and activation of JNK and P38 MAP kinase
- PMID: 19477265
- PMCID: PMC2966279
- DOI: 10.1016/j.freeradbiomed.2009.05.021
Chronic ethanol feeding potentiates Fas Jo2-induced hepatotoxicity: role of CYP2E1 and TNF-alpha and activation of JNK and P38 MAP kinase
Abstract
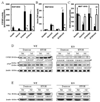
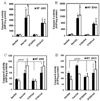
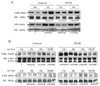
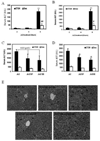
We have previously shown that treatment of mice with pyrazole or acute ethanol potentiated Fas agonistic Jo2 antibody-induced liver injury by a mechanism involving induction of CYP2E1 and elevated oxidative stress. The current study evaluated whether chronic alcohol feeding potentiates Fas-induced liver injury and whether CYP2E1 plays a role in any enhanced hepatotoxicity. Wild-type and CYP2E1 knockout mice were fed ethanol or isocaloric dextrose for 4 weeks followed by a single treatment with either saline or Jo2. Mice were killed 8 h after the Jo2 challenge. There were three- to five fold increases in transaminases and more extensive eosinophilic necrosis, hemorrhage, and infiltration of inflammatory cells in the central zone of the hepatic lobule in the ethanol-fed mice treated with Jo2 compared to the dextrose/Jo2- or ethanol/saline-treated mice. Liver injury was blunted in ethanol-fed CYP2E1 knockout mice treated with Jo2. The chronic ethanol feeding produced steatosis, elevation of CYP2E1, and oxidative stress in wild-type but not CYP2E1 knockout mice. These changes in wild-type mice fed ethanol were similar after saline or Jo2 treatment. The Jo2 treatment produced activation of JNK and P38 MAP kinase, increased activity of caspase-8 and -3, and lowered hepatic GSH levels in both the dextrose- and the alcohol-fed mice. JNK was activated at early times after Jo2 treatment in the ethanol-fed mice. Serum TNF-alpha levels were strikingly elevated in the wild-type ethanol/Jo2 group, which showed liver injury, compared to all the other groups, which did not show liver injury. Inhibition of JNK or P38 MAPK partially, but not completely, prevented the elevated liver injury in the wild-type ethanol/Jo2 mice. These results show that chronic ethanol feeding enhances Fas-induced liver injury by a mechanism associated with induction of CYP2E1, elevated serum TNF-alpha levels, and activation of MAPK.
Figures



References
-
- Albano E. Oxidative mechanisms in the pathogenesis of alcoholic liver diseases. Mol. Aspects Med. 2008;29:9–16. - PubMed
-
- Rao RK, Seth A, Sheth P. Recent Advances in Alcoholic Liver Disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am. J. Physiol. Gastrointest. Liver Physio.l. 2004;286:G881–G884. - PubMed
-
- Rouach H, Fataccioli V, Gentil M, French SW, Morimoto M, Nordmann R. Effect of chronic ethanol feeding on lipid peroxidation and protein oxidation in relation to liver pathology. Hepatology. 1997;25:351–355. - PubMed
-
- French SW, Wong K, Jui L, Albano E, Hagbjork AL, Ingelman-Sundberg M. Effect of ethanol on cytochrome P450 2E1 (CYP2E1), lipid peroxidation, and serum protein adduct formation in relation to liver pathology pathogenesis. Exp. Mol. Pathol. 1993;58:61–75. - PubMed
-
- Polavarapu R, Spitz DR, Sim JE, Follansbee MH, Oberley LW, Rahemtulla A, Nanji AA. Increased lipid peroxidation and impaired antioxidant enzyme function is associated with pathological liver injury in experimental alcoholic liver disease in rats fed diets high in corn oil and fish oil. Hepatology. 1998;27:1317–1323. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

