Expression of mutant p53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model
- PMID: 19477430
- PMCID: PMC2721466
- DOI: 10.1016/j.ccr.2009.04.001
Expression of mutant p53 proteins implicates a lineage relationship between neural stem cells and malignant astrocytic glioma in a murine model
Abstract
Recent studies have identified genes and core pathways that are altered in human glioblastoma. However, the mechanisms by which alterations of these glioblastoma genes singly and cooperatively transform brain cells remain poorly understood. Further, the cell of origin of glioblastoma is largely elusive. By targeting a p53 in-frame deletion mutation to the brain, we show that p53 deficiency provides no significant growth advantage to adult brain cells, but appears to induce pleiotropic accumulation of cooperative oncogenic alterations driving gliomagenesis. Our data show that accumulation of a detectable level of mutant p53 proteins occurs first in neural stem cells in the subventricular zone (SVZ) and that subsequent expansion of mutant p53-expressing Olig2(+) transit-amplifying progenitor-like cells in the SVZ-associated areas initiates glioma formation.
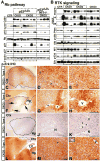
Figures







References
-
- Brannan CI, Perkins AS, Vogel KS, Ratner N, Nordlund ML, Reid SW, Buchberg AM, Jenkins NA, Parada LF, Copeland NG. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues [published erratum appears in Genes Dev 1994 Nov 15;8(22):2792] Genes & Development. 1994;8:1019–1029. - PubMed
-
- Calabrese C, Poppleton H, Kocak M, Hogg TL, Fuller C, Hamner B, Oh EY, Gaber MW, Finklestein D, Allen M, et al. A perivascular niche for brain tumor stem cells. Cancer Cell. 2007;11:69–82. - PubMed
-
- Cho Y, Gorina S, Jeffrey PD, Pavletich NP. Crystal structure of a p53 tumor suppressor-DNA complex: understanding tumorigenic mutations. Science. 1994;265:346–355. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

