Engineering of recombinant crystallization chaperones
- PMID: 19477632
- PMCID: PMC2736338
- DOI: 10.1016/j.sbi.2009.04.008
Engineering of recombinant crystallization chaperones
Abstract
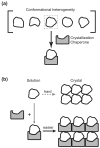
The preparation of diffraction quality crystals remains the major bottleneck in macromolecular X-ray crystallography. A crystallization chaperone is an auxiliary protein, such as fragments of monoclonal antibodies, that binds to and increases the crystallization probability of a target molecule of interest. Such chaperones reduce conformational heterogeneity, mask counterproductive surfaces while extending surfaces predisposed to forming crystal contacts, and provide phasing information. Crystallization chaperones generated using recombinant technologies have emerged as superior alternatives that increase the throughput and eliminate inherent limitations associated with antibody production by animal immunization and the hybridoma technology.
Figures


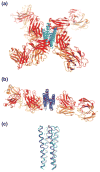
References
-
- Derewenda ZS. Rational protein crystallization by mutational surface engineering. Structure (Camb) 2004;12:529–535. - PubMed
-
- Kovari LC, Momany C, Rossmann MG. The use of antibody fragments for crystallization and structure determinations. Structure. 1995;3:1291–1293. - PubMed
-
- Iwata S, Ostermeier C, Ludwig B, Michel H. Structure at 2.8 A resolution of cytochrome c oxidase from Paracoccus denitrificans. Nature. 1995;376:660–669. - PubMed
-
- Hunte C, Michel H. Crystallisation of membrane proteins mediated by antibody fragments. Curr Opin Struct Biol. 2002;12:503–508. - PubMed
-
- Ostermeier C, Iwata S, Ludwig B, Michel H. Fv fragment-mediated crystallization of the membrane protein bacterial cytochrome c oxidase. Nat Struct Biol. 1995;2:842–846. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

