The first propeller domain of LRP6 regulates sensitivity to DKK1
- PMID: 19477926
- PMCID: PMC2719573
- DOI: 10.1091/mbc.e08-12-1252
The first propeller domain of LRP6 regulates sensitivity to DKK1
Abstract
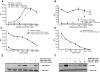
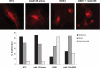
The Wnt coreceptor LRP6 is required for canonical Wnt signaling. To understand the molecular regulation of LRP6 function, we generated a series of monoclonal antibodies against the extra cellular domain (ECD) of LRP6 and selected a high-affinity mAb (mAb135) that recognizes cell surface expression of endogenous LRP6. mAb135 enhanced Wnt dependent TCF reporter activation and antagonized DKK1 dependent inhibition of Wnt3A signaling, suggesting a role in modulation of LRP6 function. Detailed analysis of LRP6 domain mutants identified Ser 243 in the first propeller domain of LRP6 as a critical residue for mAb135 binding, implicating this domain in regulating the sensitivity of LRP6 to DKK1. In agreement with this notion, mAb135 directly disrupted the interaction of DKK1 with recombinant ECD LRP6 and a truncated form of the LRP6 ECD containing only repeats 1 and 2. Finally, we found that mAb135 completely protected LRP6 from DKK1 dependent internalization. Together, these results identify the first propeller domain as a novel regulatory domain for DKK1 binding to LRP6 and show that mAb against the first propeller domain of LRP6 can be used to modulate this interaction.
Figures




References
-
- Babij P., et al. High bone mass in mice expressing a mutant LRP5 gene. J. Bone Miner. Res. 2003;18:960–974. - PubMed
-
- Baron R., Rawadi G. Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology. 2007;148:2635–2643. - PubMed
-
- Baron R., Rawadi G., Roman-Roman S. Wnt signaling: a key regulator of bone mass. Curr. Top. Dev. Biol. 2006;76:103–127. - PubMed
-
- Bhat B. M., et al. Structure-based mutation analysis shows the importance of LRP5 beta-propeller 1 in modulating Dkk1-mediated inhibition of Wnt signaling. Gene. 2007;391:103–112. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

