Cardiac fibroblast paracrine factors alter impulse conduction and ion channel expression of neonatal rat cardiomyocytes
- PMID: 19477968
- PMCID: PMC2725777
- DOI: 10.1093/cvr/cvp164
Cardiac fibroblast paracrine factors alter impulse conduction and ion channel expression of neonatal rat cardiomyocytes
Abstract
Aims: The pathological proliferation of cardiac fibroblasts (CFs) in response to heart injury results in fibrosis, which correlates with arrhythmia generation and heart failure. Here we systematically examined the effect of fibroblast-derived paracrine factors on electrical propagation in cardiomyocytes.
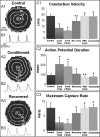
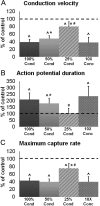
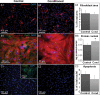
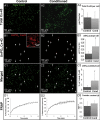
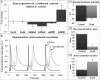
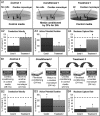
Methods and results: Neonatal rat cardiac monolayers were exposed for 24 h to media conditioned by CFs. Optical mapping, sharp microelectrode recordings, quantitative RT-PCR, and immunostaining were used to assess the changes in the propagation and shape of the action potential and underlying changes in gene and protein expression. The fibroblast paracrine factors produced a 52% reduction in cardiac conduction velocity, a 217% prolongation of action potential duration, a 64% decrease of maximum capture rate, a 21% increase in membrane resting potential, and an 80% decrease of action potential upstroke velocity. These effects were dose dependent and partially reversible with removal of the conditioned media. No fibroblast proliferation, cardiomyocyte apoptosis, or decreased connexin-43 expression, phosphorylation, and function were found in conditioned cardiac cultures. In contrast, the expression of the fast sodium, inward rectifying potassium, and transient outward potassium channels were, respectively, reduced 3.8-, 6.6-fold, and to undetectable levels. The expression of beta-myosin heavy chain increased 17.4-fold. No electrophysiological changes were observed from media conditioned by CFs in the presence of cardiomyocytes.
Conclusion: Paracrine factors from neonatal CFs alone produced significant electrophysiological changes in neonatal rat cardiomyocytes resembling those found in several cardiac pathologies.
Figures
References
-
- Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. - PubMed
-
- Nag A. Study of non-muscle cells of the adult mammalian heart: a fine structural analysis and distribution. Cytobios. 1980;28:41–61. - PubMed
-
- Banerjee I, Yekkala K, Borg TK, Baudino TA. Dynamic interactions between myocytes, fibroblasts, and extracellular matrix. Ann N Y Acad Sci. 2006;1080:76–84. - PubMed
-
- Brown R, Ambler S, Mitchell M, Long C. The cardiac fibroblast: therapeutic target in myocardial remodeling and failure. Annu Rev Pharmacol Toxicol. 2005;45:657–687. - PubMed
-
- de Bakker J, van Capelle F, Janse M, Tasseron S, Vermeulen J, de Jonge N, et al. Slow conduction in the infarcted human heart. ‘Zigzag’ course of activation. Circulation. 1993;88:915–926. - PubMed

