Stem cells are dispensable for lung homeostasis but restore airways after injury
- PMID: 19478060
- PMCID: PMC2687999
- DOI: 10.1073/pnas.0900668106
Stem cells are dispensable for lung homeostasis but restore airways after injury
Abstract
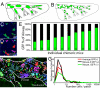
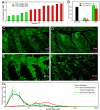
Local tissue stem cells have been described in airways of the lung but their contribution to normal epithelial maintenance is currently unknown. We therefore developed aggregation chimera mice and a whole-lung imaging method to determine the relative contributions of progenitor (Clara) and bronchiolar stem cells to epithelial maintenance and repair. In normal and moderately injured airways chimeric patches were small in size and not associated with previously described stem cell niches. This finding suggested that single, randomly distributed progenitor cells maintain normal epithelial homeostasis. In contrast we found that repair following severe lung injury resulted in the generation of rare, large clonal cell patches that were associated with stem cell niches. This study provides evidence that epithelial stem cells are dispensable for normal airway homeostasis. We also demonstrate that stem cell activation and robust clonal cellular expansion occur only during repair from severe lung injury.
Conflict of interest statement
Conflict of interest: The authors declare no conflict of interest.
Figures



References
-
- Evans MJ, Cabral-Anderson LJ, Freeman G. Role of the Clara cell in renewal of the bronchiolar epithelium. Lab Invest. 1978;38:648–653. - PubMed
-
- Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

