Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability
- PMID: 19478092
- PMCID: PMC2742868
- DOI: 10.1074/jbc.M109.016766
Occludin phosphorylation and ubiquitination regulate tight junction trafficking and vascular endothelial growth factor-induced permeability
Abstract
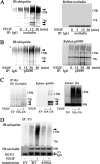
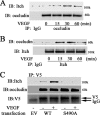
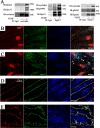
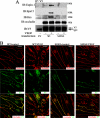
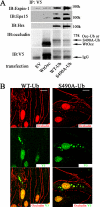
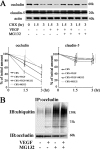
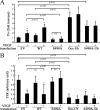
Vascular endothelial growth factor (VEGF) alters tight junctions (TJs) and promotes vascular permeability in many retinal and brain diseases. However, the molecular mechanisms of barrier regulation are poorly understood. Here we demonstrate that occludin phosphorylation and ubiquitination regulate VEGF-induced TJ protein trafficking and concomitant vascular permeability. VEGF treatment induced TJ fragmentation and occludin trafficking from the cell border to early and late endosomes, concomitant with increased occludin phosphorylation on Ser-490 and ubiquitination. Furthermore, both co-immunoprecipitation and immunocytochemistry demonstrated that VEGF treatment increased the interaction between occludin and modulators of intracellular trafficking that contain the ubiquitin interacting motif, including Epsin-1, epidermal growth factor receptor pathway substrate 15 (Eps15), and hepatocyte growth factor-regulated tyrosine kinase substrate (Hrs). Inhibiting occludin phosphorylation by mutating Ser-490 to Ala suppressed VEGF-induced ubiquitination, inhibited trafficking of TJ proteins, and prevented the increase in endothelial permeability. In addition, an occludin-ubiquitin chimera disrupted TJs and increased permeability without VEGF. These data demonstrate a novel mechanism of VEGF-induced occludin phosphorylation and ubiquitination that contributes to TJ trafficking and subsequent vascular permeability.
Figures


References
-
- Antonetti D. A., Barber A. J., Bronson S. K., Freeman W. M., Gardner T. W., Jefferson L. S., Kester M., Kimball S. R., Krady J. K., LaNoue K. F., Norbury C. C., Quinn P. G., Sandirasegarane L., Simpson I. A. (2006) Diabetes 55, 2401–2411 - PubMed
-
- Harhaj N. S., Antonetti D. A. (2004) Int. J. Biochem. Cell Biol. 36, 1206–1237 - PubMed
-
- Aiello L. P. (1997) Invest. Ophthalmol. Vis. Sci. 38, 1647–1652 - PubMed
-
- Machein M. R., Plate K. H. (2000) J. Neurooncol. 50, 109–120 - PubMed
-
- Gardner T. W., Antonetti D. A. (2008) Curr. Diab. Rep. 8, 263–269 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

