Slit diaphragms contain tight junction proteins
- PMID: 19478094
- PMCID: PMC2709684
- DOI: 10.1681/ASN.2008101117
Slit diaphragms contain tight junction proteins
Abstract
Slit diaphragms are essential components of the glomerular filtration apparatus, as changes in these junctions are the hallmark of proteinuric diseases. Slit diaphragms, considered specialized adherens junctions, contain both unique membrane proteins (e.g., nephrin, podocin, and Neph1) and typical adherens junction proteins (e.g., P-cadherin, FAT, and catenins). Whether slit diaphragms also contain tight junction proteins is unknown. Here, immunofluorescence, immunogold labeling, and cell fractionation demonstrated that rat slit diaphragms contain the tight junction proteins JAM-A (junctional adhesion molecule A), occludin, and cingulin. We found these proteins in the same protein complexes as nephrin, podocin, CD2AP, ZO-1, and Neph1 by cosedimentation, coimmunoprecipitation, and pull-down assays. PAN nephrosis increased the protein levels of JAM-A, occludin, cingulin, and ZO-1 several-fold in glomeruli and loosened their attachment to the actin cytoskeleton. These data extend current information about the molecular composition of slit diaphragms by demonstrating the presence of tight junction proteins, although slit diaphragms lack the characteristic morphologic features of tight junctions. The contribution of these proteins to the assembly of slit diaphragms and potential signaling cascades requires further investigation.
Figures
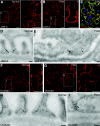
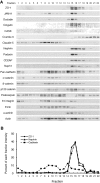


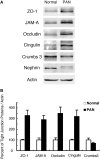
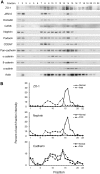


Comment in
-
How to build a tight but permeable glomerular junction.J Am Soc Nephrol. 2009 Jul;20(7):1420-1. doi: 10.1681/ASN.2009050514. Epub 2009 Jun 11. J Am Soc Nephrol. 2009. PMID: 19520750 No abstract available.
References
-
- Pavenstadt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 - PubMed
-
- Reeves W, Caulfield JP, Farquhar MG: Differentiation of epithelial foot processes and filtration slits: Sequential appearance of occluding junctions, epithelial polyanion, and slit membranes in developing glomeruli. Lab Invest 39: 90–100, 1978 - PubMed
-
- Caulfield JP, Reid JJ, Farquhar MG: Alterations of the glomerular epithelium in acute aminonucleoside nephrosis. Evidence for formation of occluding junctions and epithelial cell detachment. Lab Invest 34: 43–59, 1976 - PubMed
-
- Kurihara H, Anderson JM, Farquhar MG: Increased Tyr phosphorylation of ZO-1 during modification of tight junctions between glomerular foot processes. Am J Physiol 268: F514–524, 1995 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

