Acute inhibition of Ca2+/calmodulin-dependent protein kinase II reverses experimental neuropathic pain in mice
- PMID: 19478130
- PMCID: PMC2713096
- DOI: 10.1124/jpet.109.152165
Acute inhibition of Ca2+/calmodulin-dependent protein kinase II reverses experimental neuropathic pain in mice
Abstract
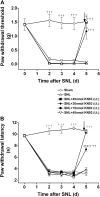
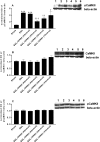
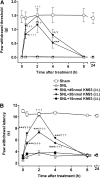
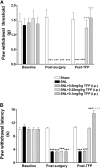
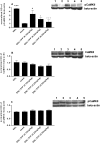
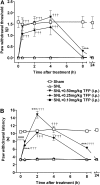
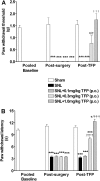
The limited data that currently exist for the role of Ca(2+)/calmodulin-dependent protein kinase II (CaMKII) in neuropathic pain are conflicting. In the present study, we tested the hypothesis that CaMKII is required for the maintenance of neuropathic pain in a rodent model of experimental mononeuropathy. Spinal nerve L(5)/L(6) ligation (SNL) was found to increase the spinal activity of CaMKII (pCaMKII) on the ipsilateral (but not contralateral) side. This effect was blocked by 2-[N-(2-hydroxyethyl)-N-(4-methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine) (KN93) (intrathecal injection), a CaMKII inhibitor. Acute treatment with KN93 dose-dependently reversed SNL-induced thermal hyperalgesia and mechanical allodynia. The action of KN93 lasted for at least 2 to 4 h. 2-[N-(4-Methoxybenzenesulfonyl)]amino-N-(4-chlorocinnamyl)-N-methylbenzylamine (KN92) (45 nmol i.t.), an inactive analog of KN93, showed no effect on SNL-induced CaMKII activation, allodynia, or hyperalgesia. We further examined the pharmacologic action of trifluoperazine, a clinically used antipsychotic drug that we found to be a potent CaMKII inhibitor in these assays. Trifluoperazine (administered intraperitoneally or by mouth) dose-dependently reversed SNL-induced mechanical allodynia, thermal hyperalgesia, and CaMKII activation without causing locomotor impairment in mice at the highest doses used. In conclusion, our findings support a critical role of CaMKII in neuropathic pain. Blocking CaMKII or CaMKII-mediated signaling may offer a novel therapeutic target for the treatment of neuropathic pain.
Figures

References
-
- Brüggemann I, Schulz S, Wiborny D, and Höllt V (2000) Colocalization of the mu-opioid receptor and calcium/calmodulin-dependent kinase II in distinct pain-processing brain regions. Brain Res Mol Brain Res 85 239-250. - PubMed
-
- Carlton SM (2002) Localization of CaMKIIalpha in rat primary sensory neurons: increase in inflammation. Brain Res 947 252-259. - PubMed
-
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, and Yaksh TL (1994) Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods 53 55-63. - PubMed
-
- Choi SS, Seo YJ, Shim EJ, Kwon MS, Lee JY, Ham YO, and Suh HW (2006) Involvement of phosphorylated Ca2+/calmodulin-dependent protein kinase II and phosphorylated extracellular signal-regulated protein in the mouse formalin pain model. Brain Res 1108 28-38. - PubMed
-
- Dai Y, Wang H, Ogawa A, Yamanaka H, Obata K, Tokunaga A, and Noguchi K (2005) Ca2+/calmodulin-dependent protein kinase II in the spinal cord contributes to neuropathic pain in a rat model of mononeuropathy. Eur J Neurosci 21 2467-2474. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

