Nuclear PTEN levels and G2 progression in melanoma cells
- PMID: 19478684
- PMCID: PMC2750820
- DOI: 10.1097/CMR.0b013e32832ccd6e
Nuclear PTEN levels and G2 progression in melanoma cells
Abstract
The phosphatase and tensin homolog (PTEN) exerts its function, in part, by negatively regulating the well-known phosphatidylinositol-3-kinase/AKT signaling pathway. Previous histological work has suggested that alterations in the nuclear/cytoplasmic compartmentalization of PTEN may play a role in the development and progression of melanoma. In this study, we examined the nuclear/cytoplasmic compartmentalization of PTEN in melanoma cell lines and its correlation with the cell cycle. Studies were performed in melanoma cells lines using classic cell biological techniques. In contrast to breast cancer cell lines, we found that increased levels of nuclear PTEN levels correlate with G2 rather than with G1 arrest. In WM164 and SKmel28 cells, overexpression of PTEN protein did not significantly increase the number of cells in the G2 phase. Differential CDC2 phosphorylation levels in cells that overexpressed PTEN compared with those where PTEN was downregulated suggest some involvement of PTEN in G2 checkpoint regulation. The data suggest that although nuclear PTEN levels correlate with the G2 phase, the role of PTEN in modulating G2/M arrest is not limiting. Further, the specific cell cycle phase regulated by nuclear PTEN is cell-type dependent. Taken together, our observations suggest that in melanoma, nuclear PTEN is involved in G2 progression possibly through the modulation of CDC2, opening up a new arena for investigation.
Conflict of interest statement
Conflict of interest: All of the authors declare no conflict of interest.
Figures
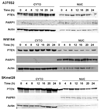




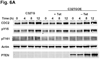

References
-
- Eng C. PTEN: one gene, many syndromes. Hum Mutat. 2003;22:183–198. - PubMed
-
- Liaw D, Marsh DJ, Li J, Dahia PL, Wang SI, Zheng Z, et al. Germline mutations of the PTEN gene in Cowden disease, an inherited breast and thyroid cancer syndrome. Nat Genet. 1997;16:64–67. - PubMed
-
- Marsh DJ, Dahia PL, Zheng Z, Liaw D, Parsons R, Gorlin RJ, et al. Germline mutations in PTEN are present in Bannayan-Zonana syndrome. Nat Genet. 1997;16:333–334. - PubMed
-
- Eng C, Thiele H, Zhou XP, Gorlin RJ, Hennekam RC, Winter RM. PTEN mutations and proteus syndrome. Lancet. 2001;358:2079–2080. - PubMed
-
- Zhou XP, Marsh DJ, Hampel H, Mulliken JB, Gimm O, Eng C. Germline and germline mosaic PTEN mutations associated with a Proteus-like syndrome of hemihypertrophy, lower limb asymmetry, arteriovenous malformations and lipomatosis. Hum Mol Genet. 2000;9:765–768. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

