Transposon-mediated genome manipulation in vertebrates
- PMID: 19478801
- PMCID: PMC2867038
- DOI: 10.1038/nmeth.1332
Transposon-mediated genome manipulation in vertebrates
Abstract
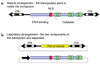
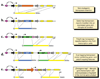
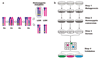
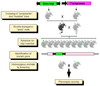
Transposable elements are DNA segments with the unique ability to move about in the genome. This inherent feature can be exploited to harness these elements as gene vectors for genome manipulation. Transposon-based genetic strategies have been established in vertebrate species over the last decade, and current progress in this field suggests that transposable elements will serve as indispensable tools. In particular, transposons can be applied as vectors for somatic and germline transgenesis, and as insertional mutagens in both loss-of-function and gain-of-function forward mutagenesis screens. In addition, transposons will gain importance in future cell-based clinical applications, including nonviral gene transfer into stem cells and the rapidly developing field of induced pluripotent stem cells. Here we provide an overview of transposon-based methods used in vertebrate model organisms with an emphasis on the mouse system and highlight the most important considerations concerning genetic applications of the transposon systems.
Figures


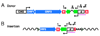
Similar articles
-
Technology transfer from worms and flies to vertebrates: transposition-based genome manipulations and their future perspectives.Genome Biol. 2007;8 Suppl 1(Suppl 1):S1. doi: 10.1186/gb-2007-8-s1-s1. Genome Biol. 2007. PMID: 18047686 Free PMC article. Review.
-
Efficient transposition of the piggyBac (PB) transposon in mammalian cells and mice.Cell. 2005 Aug 12;122(3):473-83. doi: 10.1016/j.cell.2005.07.013. Cell. 2005. PMID: 16096065
-
Generating and manipulating transgenic animals using transposable elements.Reprod Biol Endocrinol. 2003 Nov 7;1:80. doi: 10.1186/1477-7827-1-80. Reprod Biol Endocrinol. 2003. PMID: 14613544 Free PMC article. Review.
-
Contemporary Transposon Tools: A Review and Guide through Mechanisms and Applications of Sleeping Beauty, piggyBac and Tol2 for Genome Engineering.Int J Mol Sci. 2021 May 11;22(10):5084. doi: 10.3390/ijms22105084. Int J Mol Sci. 2021. PMID: 34064900 Free PMC article. Review.
-
Transposon vectors for gene-trap insertional mutagenesis in vertebrates.Genesis. 2004 Aug;39(4):225-33. doi: 10.1002/gene.20049. Genesis. 2004. PMID: 15286994
Cited by
-
Genetic association and characterization of FSTL5 in isolated clubfoot.Hum Mol Genet. 2021 Jan 21;29(22):3717-3728. doi: 10.1093/hmg/ddaa236. Hum Mol Genet. 2021. PMID: 33105483 Free PMC article.
-
Genomic methods in profiling DNA accessibility and factor localization.Chromosome Res. 2020 Mar;28(1):69-85. doi: 10.1007/s10577-019-09619-9. Epub 2019 Nov 27. Chromosome Res. 2020. PMID: 31776829 Free PMC article.
-
First full views of a CRISPR-guided system for gene insertion.Nature. 2023 Jan;613(7945):634-635. doi: 10.1038/d41586-022-04584-6. Nature. 2023. PMID: 36631579 No abstract available.
-
Evolution-guided evaluation of the inverted terminal repeats of the synthetic transposon Sleeping Beauty.Sci Rep. 2019 Feb 4;9(1):1171. doi: 10.1038/s41598-018-38061-w. Sci Rep. 2019. PMID: 30718656 Free PMC article.
-
At the Intersection of Biomaterials and Gene Therapy: Progress in Non-viral Delivery of Nucleic Acids.Front Bioeng Biotechnol. 2019 Jun 4;7:131. doi: 10.3389/fbioe.2019.00131. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 31214586 Free PMC article. Review.
References
-
- Lander ES, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. - PubMed
-
- Feng Q, Moran JV, Kazazian HH, Jr, Boeke JD. Human L1 retrotransposon encodes a conserved endonuclease required for retrotransposition. Cell. 1996;87:905–916. - PubMed
-
- Mathias SL, Scott AF, Kazazian HH, Jr, Boeke JD, Gabriel A. Reverse transcriptase encoded by a human transposable element. Science. 1991;254:1808–1810. - PubMed
-
- Luan DD, Korman MH, Jakubczak JL, Eickbush TH. Reverse transcription of R2Bm RNA is primed by a nick at the chromosomal target site: a mechanism for non-LTR retrotransposition. Cell. 1993;72:595–605. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

