Combination therapy using chimeric monoclonal antibodies protects mice from lethal H5N1 infection and prevents formation of escape mutants
- PMID: 19478856
- PMCID: PMC2682562
- DOI: 10.1371/journal.pone.0005672
Combination therapy using chimeric monoclonal antibodies protects mice from lethal H5N1 infection and prevents formation of escape mutants
Abstract
Background: Given that there is a possibility of a human H5N1 pandemic and the fact that the recent H5N1 viruses are resistant to the anti-viral drugs, newer strategies for effective therapy are warranted. Previous studies show that single mAbs in immune prophylaxis can be protective against H5N1 infection. But a single mAb may not be effective in neutralization of a broad range of different strains of H5N1 and control of potential neutralization escape mutants.
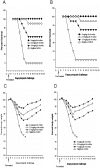
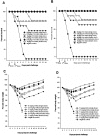
Methods/principal findings: We selected two mAbs which recognized different epitopes on the hemagglutinin molecule. These two mAbs could each neutralize in vitro escape mutants to the other and in combination could effectively neutralize viruses from clades 0, 1, 2.1, 2.2, 2.3, 4, 7 and 8 of influenza A H5N1 viruses. This combination of chimeric mAbs when administered passively, pre or post challenge with 10 MLD50 (50% mouse lethal dose) HPAI H5N1 influenza A viruses could protect 100% of the mice from two different clades of viruses (clades 1 and 2.1). We also tested the efficacy of a single dose of the combination of mAbs versus two doses. Two doses of the combination therapy not only affected early clearance of the virus from the lung but could completely prevent lung pathology of the H5N1 infected mice. No escape variants were detected after therapy.
Conclusions/significance: Our studies provide proof of concept that the synergistic action of two or more mAbs in combination is required for preventing the generation of escape mutants and also to enhance the therapeutic efficacy of passive therapy against H5N1 infection. Combination therapy may allow for a lower dose of antibody to be administered for passive therapy of influenza infection and hence can be made available at reduced economic costs during an outbreak.
Conflict of interest statement
Figures



References
-
- Veits J, Romer-Oberdorfer A, Helferich D, Durban M, Suezer Y, et al. Protective efficacy of several vaccines against highly pathogenic H5N1 avian influenza virus under experimental conditions. Vaccine. 2008;26:1688–1696. - PubMed
-
- Li KS, Guan Y, Wang J, Smith GJ, Xu KM, et al. Genesis of a highly pathogenic and potentially pandemic H5N1 influenza virus in eastern Asia. Nature. 2004;430:209–213. - PubMed
-
- Le QM, Kiso M, Someya K, Sakai YT, Nguyen TH, et al. Avian flu: Isolation of drug-resistant H5N1 virus. Nature. 2005;437:1108. - PubMed
-
- Casadevall A, Dadachova E, Pirofski L. Passive antibody therapy for infectious diseases. Nat Rev Microbiol. 2004;2:695–703. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

