A therapeutic antibody against west nile virus neutralizes infection by blocking fusion within endosomes
- PMID: 19478866
- PMCID: PMC2679195
- DOI: 10.1371/journal.ppat.1000453
A therapeutic antibody against west nile virus neutralizes infection by blocking fusion within endosomes
Abstract
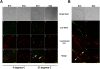
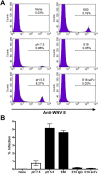
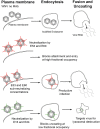
Defining the precise cellular mechanisms of neutralization by potently inhibitory antibodies is important for understanding how the immune system successfully limits viral infections. We recently described a potently inhibitory monoclonal antibody (MAb E16) against the envelope (E) protein of West Nile virus (WNV) that neutralizes infection even after virus has spread to the central nervous system. Herein, we define its mechanism of inhibition. E16 blocks infection primarily at a post-attachment step as antibody-opsonized WNV enters permissive cells but cannot escape from endocytic compartments. These cellular experiments suggest that E16 blocks the acid-catalyzed fusion step that is required for nucleocapsid entry into the cytoplasm. Indeed, E16 directly inhibits fusion of WNV with liposomes. Additionally, low-pH exposure of E16-WNV complexes in the absence of target membranes did not fully inactivate infectious virus, further suggesting that E16 prevents a structural transition required for fusion. Thus, a strongly neutralizing anti-WNV MAb with therapeutic potential is potently inhibitory because it blocks viral fusion and thereby promotes clearance by delivering virus to the lysosome for destruction.
Conflict of interest statement
MSD is a consultant for MacroGenics, which has licensed the E16 antibody for possible commercial use.
Figures

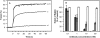

Similar articles
-
A protective human monoclonal antibody targeting the West Nile virus E protein preferentially recognizes mature virions.Nat Microbiol. 2019 Jan;4(1):71-77. doi: 10.1038/s41564-018-0283-7. Epub 2018 Nov 19. Nat Microbiol. 2019. PMID: 30455471 Free PMC article.
-
Neutralization of West Nile virus by cross-linking of its surface proteins with Fab fragments of the human monoclonal antibody CR4354.Proc Natl Acad Sci U S A. 2010 Nov 2;107(44):18950-5. doi: 10.1073/pnas.1011036107. Epub 2010 Oct 18. Proc Natl Acad Sci U S A. 2010. PMID: 20956322 Free PMC article.
-
Structural basis of West Nile virus neutralization by a therapeutic antibody.Nature. 2005 Sep 29;437(7059):764-9. doi: 10.1038/nature03956. Nature. 2005. PMID: 16193056 Free PMC article.
-
The molecular basis of antibody-mediated neutralization of West Nile virus.Expert Opin Biol Ther. 2007 Jun;7(6):885-92. doi: 10.1517/14712598.7.6.885. Expert Opin Biol Ther. 2007. PMID: 17555373 Review.
-
The molecular basis of antibody protection against West Nile virus.Curr Top Microbiol Immunol. 2008;317:125-53. doi: 10.1007/978-3-540-72146-8_5. Curr Top Microbiol Immunol. 2008. PMID: 17990792 Review.
Cited by
-
The Japanese Encephalitis Antigenic Complex Viruses: From Structure to Immunity.Viruses. 2022 Oct 8;14(10):2213. doi: 10.3390/v14102213. Viruses. 2022. PMID: 36298768 Free PMC article. Review.
-
Enhancement of anti-DIII antibodies by the C3d derivative P28 results in lower viral titers and augments protection in mice.Virol J. 2010 May 12;7:95. doi: 10.1186/1743-422X-7-95. Virol J. 2010. PMID: 20462412 Free PMC article.
-
Mechanism of differential Zika and dengue virus neutralization by a public antibody lineage targeting the DIII lateral ridge.J Exp Med. 2020 Feb 3;217(2):e20191792. doi: 10.1084/jem.20191792. J Exp Med. 2020. PMID: 31757867 Free PMC article.
-
Human IgG subclasses: in vitro neutralization of and in vivo protection against West Nile virus.J Virol. 2011 Feb;85(4):1896-9. doi: 10.1128/JVI.02155-10. Epub 2010 Dec 1. J Virol. 2011. PMID: 21123389 Free PMC article.
-
The domain I-domain III linker plays an important role in the fusogenic conformational change of the alphavirus membrane fusion protein.J Virol. 2011 Jul;85(13):6334-42. doi: 10.1128/JVI.00596-11. Epub 2011 May 4. J Virol. 2011. PMID: 21543498 Free PMC article.
References
-
- Reading SA, Dimmock NJ. Neutralization of animal virus infectivity by antibody. Arch Virol. 2007;152:1047–1059. - PubMed
-
- Brinton MA. The molecular biology of West Nile Virus: a new invader of the western hemisphere. Annu Rev Microbiol. 2002;56:371–402. - PubMed
-
- Lindenbach BD, Rice CM. Flaviviridae: The viruses and their replication. In: Knipe DM, Howley PM, editors. Fields Virology. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 991–1041.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

