Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking
- PMID: 19478868
- PMCID: PMC2679223
- DOI: 10.1371/journal.ppat.1000450
Vpu antagonizes BST-2-mediated restriction of HIV-1 release via beta-TrCP and endo-lysosomal trafficking
Abstract
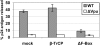
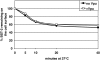
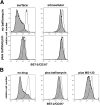
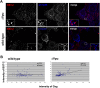
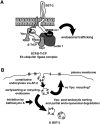
The interferon-induced transmembrane protein BST-2/CD317 (tetherin) restricts the release of diverse enveloped viruses from infected cells. The HIV-1 accessory protein Vpu antagonizes this restriction by an unknown mechanism that likely involves the down-regulation of BST-2 from the cell surface. Here, we show that the optimal removal of BST-2 from the plasma membrane by Vpu requires the cellular protein beta-TrCP, a substrate adaptor for a multi-subunit SCF E3 ubiquitin ligase complex and a known Vpu-interacting protein. beta-TrCP is also required for the optimal enhancement of virion-release by Vpu. Mutations in the DSGxxS beta-TrCP binding-motif of Vpu impair both the down-regulation of BST-2 and the enhancement of virion-release. Such mutations also confer dominant-negative activity, consistent with a model in which Vpu links BST-2 to beta-TrCP. Optimal down-regulation of BST-2 from the cell surface by Vpu also requires the endocytic clathrin adaptor AP-2, although the rate of endocytosis is not increased; these data suggest that Vpu induces post-endocytic membrane trafficking events whose net effect is the removal of BST-2 from the cell surface. In addition to its marked effect on cell-surface levels, Vpu modestly decreases the total cellular levels of BST-2. The decreases in cell-surface and intracellular BST-2 are inhibited by bafilomycin A1, an inhibitor of endosomal acidification; these data suggest that Vpu induces late endosomal targeting and partial degradation of BST-2 in lysosomes. The Vpu-mediated decrease in surface expression is associated with reduced co-localization of BST-2 and the virion protein Gag along the plasma membrane. Together, the data support a model in which Vpu co-opts the beta-TrCP/SCF E3 ubiquitin ligase complex to induce endosomal trafficking events that remove BST-2 from its site of action as a virion-tethering factor.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Vpu directs the degradation of the human immunodeficiency virus restriction factor BST-2/Tetherin via a {beta}TrCP-dependent mechanism.J Virol. 2009 Aug;83(16):7931-47. doi: 10.1128/JVI.00242-09. Epub 2009 Jun 10. J Virol. 2009. PMID: 19515779 Free PMC article.
-
β-TrCP is dispensable for Vpu's ability to overcome the CD317/Tetherin-imposed restriction to HIV-1 release.Retrovirology. 2011 Feb 10;8:9. doi: 10.1186/1742-4690-8-9. Retrovirology. 2011. PMID: 21310048 Free PMC article.
-
HIV-1 accessory protein Vpu internalizes cell-surface BST-2/tetherin through transmembrane interactions leading to lysosomes.J Biol Chem. 2009 Dec 11;284(50):35060-72. doi: 10.1074/jbc.M109.058305. Epub 2009 Oct 16. J Biol Chem. 2009. PMID: 19837671 Free PMC article.
-
Interactions of viral protein U (Vpu) with cellular factors.Curr Top Microbiol Immunol. 2009;339:27-45. doi: 10.1007/978-3-642-02175-6_2. Curr Top Microbiol Immunol. 2009. PMID: 20012522 Review.
-
β-TrCP dependency of HIV-1 Vpu-induced downregulation of CD4 and BST-2/tetherin.Curr HIV Res. 2012 Jun;10(4):307-14. doi: 10.2174/157016212800792441. Curr HIV Res. 2012. PMID: 22524179 Review.
Cited by
-
HIV-1 Vpu affects the anterograde transport and the glycosylation pattern of NTB-A.Virology. 2013 Jun 5;440(2):190-203. doi: 10.1016/j.virol.2013.02.021. Epub 2013 Mar 22. Virology. 2013. PMID: 23528733 Free PMC article.
-
HSV-2 glycoprotein gD targets the CC domain of tetherin and promotes tetherin degradation via lysosomal pathway.Virol J. 2016 Sep 15;13(1):154. doi: 10.1186/s12985-016-0610-7. Virol J. 2016. PMID: 27630089 Free PMC article.
-
Antagonism to human BST-2/tetherin by Sendai virus glycoproteins.J Gen Virol. 2013 Jun;94(Pt 6):1211-1219. doi: 10.1099/vir.0.051771-0. Epub 2013 Mar 6. J Gen Virol. 2013. PMID: 23468424 Free PMC article.
-
Determinants in HIV-2 Env and tetherin required for functional interaction.Retrovirology. 2015 Aug 7;12:67. doi: 10.1186/s12977-015-0194-0. Retrovirology. 2015. PMID: 26248668 Free PMC article.
-
The Tetherin Antagonism of the Ebola Virus Glycoprotein Requires an Intact Receptor-Binding Domain and Can Be Blocked by GP1-Specific Antibodies.J Virol. 2016 Nov 28;90(24):11075-11086. doi: 10.1128/JVI.01563-16. Print 2016 Dec 15. J Virol. 2016. PMID: 27707924 Free PMC article.
References
-
- Malim MH, Emerman M. HIV-1 accessory proteins–ensuring viral survival in a hostile environment. Cell Host Microbe. 2008;3:388–398. - PubMed
-
- Yu X, Yu Y, Liu B, Luo K, Kong W, et al. Induction of APOBEC3G ubiquitination and degradation by an HIV-1 Vif-Cul5-SCF complex. Science. 2003;302:1056–1060. - PubMed
-
- Harris RS, Bishop KN, Sheehy AM, Craig HM, Petersen-Mahrt SK, et al. DNA deamination mediates innate immunity to retroviral infection. Cell. 2003;113:803–809. - PubMed
-
- Neil SJ, Zang T, Bieniasz PD. Tetherin inhibits retrovirus release and is antagonized by HIV-1 Vpu. Nature. 2008;451:425–430. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

