The heart is an early target of anthrax lethal toxin in mice: a protective role for neuronal nitric oxide synthase (nNOS)
- PMID: 19478875
- PMCID: PMC2680977
- DOI: 10.1371/journal.ppat.1000456
The heart is an early target of anthrax lethal toxin in mice: a protective role for neuronal nitric oxide synthase (nNOS)
Abstract
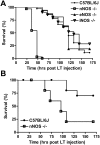
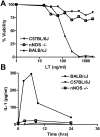
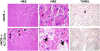
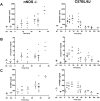
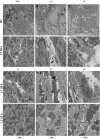
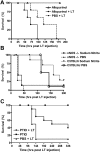
Anthrax lethal toxin (LT) induces vascular insufficiency in experimental animals through unknown mechanisms. In this study, we show that neuronal nitric oxide synthase (nNOS) deficiency in mice causes strikingly increased sensitivity to LT, while deficiencies in the two other NOS enzymes (iNOS and eNOS) have no effect on LT-mediated mortality. The increased sensitivity of nNOS-/- mice was independent of macrophage sensitivity to toxin, or cytokine responses, and could be replicated in nNOS-sufficient wild-type (WT) mice through pharmacological inhibition of the enzyme with 7-nitroindazole. Histopathological analyses showed that LT induced architectural changes in heart morphology of nNOS-/- mice, with rapid appearance of novel inter-fiber spaces but no associated apoptosis of cardiomyocytes. LT-treated WT mice had no histopathology observed at the light microscopy level. Electron microscopic analyses of LT-treated mice, however, revealed striking pathological changes in the hearts of both nNOS-/- and WT mice, varying only in severity and timing. Endothelial/capillary necrosis and degeneration, inter-myocyte edema, myofilament and mitochondrial degeneration, and altered sarcoplasmic reticulum cisternae were observed in both LT-treated WT and nNOS-/- mice. Furthermore, multiple biomarkers of cardiac injury (myoglobin, cardiac troponin-I, and heart fatty acid binding protein) were elevated in LT-treated mice very rapidly (by 6 h after LT injection) and reached concentrations rarely reported in mice. Cardiac protective nitrite therapy and allopurinol therapy did not have beneficial effects in LT-treated mice. Surprisingly, the potent nitric oxide scavenger, carboxy-PTIO, showed some protective effect against LT. Echocardiography on LT-treated mice indicated an average reduction in ejection fraction following LT treatment in both nNOS-/- and WT mice, indicative of decreased contractile function in the heart. We report the heart as an early target of LT in mice and discuss a protective role for nNOS against LT-mediated cardiac damage.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
The opposite roles of nNOS in cardiac ischemia-reperfusion-induced injury and in ischemia preconditioning-induced cardioprotection in mice.J Physiol Sci. 2009 Jul;59(4):253-62. doi: 10.1007/s12576-009-0030-1. Epub 2009 Mar 4. J Physiol Sci. 2009. PMID: 19340535 Free PMC article.
-
Reduced phospholamban phosphorylation is associated with impaired relaxation in left ventricular myocytes from neuronal NO synthase-deficient mice.Circ Res. 2008 Feb 1;102(2):242-9. doi: 10.1161/CIRCRESAHA.107.164798. Epub 2007 Nov 15. Circ Res. 2008. PMID: 18007024
-
Displacement-encoded and manganese-enhanced cardiac MRI reveal that nNOS, not eNOS, plays a dominant role in modulating contraction and calcium influx in the mammalian heart.Am J Physiol Heart Circ Physiol. 2012 Jan;302(2):H412-9. doi: 10.1152/ajpheart.00705.2011. Epub 2011 Nov 4. Am J Physiol Heart Circ Physiol. 2012. PMID: 22058155 Free PMC article.
-
Neuronal and endothelial nitric oxide synthase gene knockout mice.Braz J Med Biol Res. 1999 Nov;32(11):1353-9. doi: 10.1590/s0100-879x1999001100005. Braz J Med Biol Res. 1999. PMID: 10559836 Review.
-
Role of nNOS in cardiac ischemia-reperfusion injury.Trends Cardiovasc Med. 2011 Feb;21(2):58-63. doi: 10.1016/j.tcm.2012.03.001. Trends Cardiovasc Med. 2011. PMID: 22578242 Review.
Cited by
-
The biological activity of auranofin: implications for novel treatment of diseases.Inflammopharmacology. 2012 Dec;20(6):297-306. doi: 10.1007/s10787-012-0149-1. Epub 2012 Sep 11. Inflammopharmacology. 2012. PMID: 22965242 Review.
-
Anthrax lethal toxin induces acute diastolic dysfunction in rats through disruption of the phospholamban signaling network.Int J Cardiol. 2013 Oct 9;168(4):3884-95. doi: 10.1016/j.ijcard.2013.06.050. Epub 2013 Jul 30. Int J Cardiol. 2013. PMID: 23907041 Free PMC article.
-
The effect of Ethanolic extract of Indonesian propolis on endothelial dysfunction and Multi Organ dysfunction syndrome in anthrax animal model.Saudi J Biol Sci. 2022 Feb;29(2):1118-1124. doi: 10.1016/j.sjbs.2021.09.054. Epub 2021 Sep 23. Saudi J Biol Sci. 2022. PMID: 35197781 Free PMC article. No abstract available.
-
Small-molecule inhibitors of lethal factor protease activity protect against anthrax infection.Antimicrob Agents Chemother. 2013 Sep;57(9):4139-45. doi: 10.1128/AAC.00941-13. Epub 2013 Jun 17. Antimicrob Agents Chemother. 2013. PMID: 23774434 Free PMC article.
-
Cardiac-specific catalase overexpression rescues anthrax lethal toxin-induced cardiac contractile dysfunction: role of oxidative stress and autophagy.BMC Med. 2012 Nov 7;10:134. doi: 10.1186/1741-7015-10-134. BMC Med. 2012. PMID: 23134810 Free PMC article.
References
-
- Leppla SH. Bacillus anthracis toxins. In: Alouf JE, Popoff MR, editors. The Comprehensive Sourcebook of Bacterial Protein Toxins. Burlington, MA: Academic Press; 2006. pp. 323–347.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

