Mycobacterium tuberculosis universal stress protein Rv2623 regulates bacillary growth by ATP-Binding: requirement for establishing chronic persistent infection
- PMID: 19478878
- PMCID: PMC2682197
- DOI: 10.1371/journal.ppat.1000460
Mycobacterium tuberculosis universal stress protein Rv2623 regulates bacillary growth by ATP-Binding: requirement for establishing chronic persistent infection
Erratum in
- PLoS Pathog. 2009 Sep;5(9). doi: 10.1371/annotation/2b1a4b06-9558-448b-a8e8-5e2d407816a0
Abstract
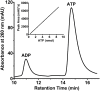
Tuberculous latency and reactivation play a significant role in the pathogenesis of tuberculosis, yet the mechanisms that regulate these processes remain unclear. The Mycobacterium tuberculosisuniversal stress protein (USP) homolog, rv2623, is among the most highly induced genes when the tubercle bacillus is subjected to hypoxia and nitrosative stress, conditions thought to promote latency. Induction of rv2623 also occurs when M. tuberculosis encounters conditions associated with growth arrest, such as the intracellular milieu of macrophages and in the lungs of mice with chronic tuberculosis. Therefore, we tested the hypothesis that Rv2623 regulates tuberculosis latency. We observed that an Rv2623-deficient mutant fails to establish chronic tuberculous infection in guinea pigs and mice, exhibiting a hypervirulence phenotype associated with increased bacterial burden and mortality. Consistent with this in vivo growth-regulatory role, constitutive overexpression of rv2623 attenuates mycobacterial growth in vitro. Biochemical analysis of purified Rv2623 suggested that this mycobacterial USP binds ATP, and the 2.9-A-resolution crystal structure revealed that Rv2623 engages ATP in a novel nucleotide-binding pocket. Structure-guided mutagenesis yielded Rv2623 mutants with reduced ATP-binding capacity. Analysis of mycobacteria overexpressing these mutants revealed that the in vitro growth-inhibitory property of Rv2623 correlates with its ability to bind ATP. Together, the results indicate that i) M. tuberculosis Rv2623 regulates mycobacterial growth in vitro and in vivo, and ii) Rv2623 is required for the entry of the tubercle bacillus into the chronic phase of infection in the host; in addition, iii) Rv2623 binds ATP; and iv) the growth-regulatory attribute of this USP is dependent on its ATP-binding activity. We propose that Rv2623 may function as an ATP-dependent signaling intermediate in a pathway that promotes persistent infection.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




Similar articles
-
Mycobacterium tuberculosis universal stress protein Rv2623 interacts with the putative ATP binding cassette (ABC) transporter Rv1747 to regulate mycobacterial growth.PLoS Pathog. 2017 Jul 28;13(7):e1006515. doi: 10.1371/journal.ppat.1006515. eCollection 2017 Jul. PLoS Pathog. 2017. PMID: 28753640 Free PMC article.
-
Identification and characterization of mycobacterial proteins differentially expressed under standing and shaking culture conditions, including Rv2623 from a novel class of putative ATP-binding proteins.Infect Immun. 2001 Sep;69(9):5777-85. doi: 10.1128/IAI.69.9.5777-5785.2001. Infect Immun. 2001. PMID: 11500455 Free PMC article.
-
Responses of Mycobacterium tuberculosis hemoglobin promoters to in vitro and in vivo growth conditions.Appl Environ Microbiol. 2008 Jun;74(11):3512-22. doi: 10.1128/AEM.02663-07. Epub 2008 Apr 4. Appl Environ Microbiol. 2008. PMID: 18390674 Free PMC article.
-
Unique structural and mechanistic properties of mycobacterial F-ATP synthases: Implications for drug design.Prog Biophys Mol Biol. 2020 May;152:64-73. doi: 10.1016/j.pbiomolbio.2019.11.006. Epub 2019 Nov 16. Prog Biophys Mol Biol. 2020. PMID: 31743686 Review.
-
Universal stress proteins and Mycobacterium tuberculosis.Res Microbiol. 2003 Jul-Aug;154(6):387-92. doi: 10.1016/S0923-2508(03)00081-0. Res Microbiol. 2003. PMID: 12892844 Review.
Cited by
-
Analysis of the Staphylococcus aureus abscess proteome identifies antimicrobial host proteins and bacterial stress responses at the host-pathogen interface.Pathog Dis. 2013 Oct;69(1):36-48. doi: 10.1111/2049-632X.12063. Epub 2013 Oct 1. Pathog Dis. 2013. PMID: 23847107 Free PMC article.
-
Characterization of a cAMP responsive transcription factor, Cmr (Rv1675c), in TB complex mycobacteria reveals overlap with the DosR (DevR) dormancy regulon.Nucleic Acids Res. 2016 Jan 8;44(1):134-51. doi: 10.1093/nar/gkv889. Epub 2015 Sep 10. Nucleic Acids Res. 2016. PMID: 26358810 Free PMC article.
-
Francisella tularensis universal stress protein contributes to persistence during growth arrest and paraquat-induced superoxide stress.J Bacteriol. 2025 Feb 20;207(2):e0037724. doi: 10.1128/jb.00377-24. Epub 2025 Jan 23. J Bacteriol. 2025. PMID: 39846732 Free PMC article.
-
Vaccination with BCGΔBCG1419c protects against pulmonary and extrapulmonary TB and is safer than BCG.Sci Rep. 2021 Jun 14;11(1):12417. doi: 10.1038/s41598-021-91993-8. Sci Rep. 2021. PMID: 34127755 Free PMC article.
-
Individual Mycobacterium tuberculosis universal stress protein homologues are dispensable in vitro.Tuberculosis (Edinb). 2010 Jul;90(4):236-44. doi: 10.1016/j.tube.2010.03.013. Epub 2010 Jun 11. Tuberculosis (Edinb). 2010. PMID: 20541977 Free PMC article.
References
-
- WHO. Global Tuberculosis Control: Surveillance, Planning, and Financing (2007.378) Geneva, Switzerland: World Health Organization Press; 2007.
-
- Flynn JL, Chan J. Immunology of tuberculosis. Annu Rev Immunol. 2001;19:93–129. - PubMed
-
- Ohno H, Zhu G, Mohan VP, Chu D, Kohno S, et al. The effects of reactive nitrogen intermediates on gene expression in Mycobacterium tuberculosis. Cell Microbiol. 2003;5:637–648. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- HHSN266200400091C/AI/NIAID NIH HHS/United States
- S10 RR015859/RR/NCRR NIH HHS/United States
- R01 HL071241/HL/NHLBI NIH HHS/United States
- P30 AI051519/AI/NIAID NIH HHS/United States
- R56 AI007289/AI/NIAID NIH HHS/United States
- T32 AI007501/AI/NIAID NIH HHS/United States
- P30 EB009998/EB/NIBIB NIH HHS/United States
- R01 GM54469/GM/NIGMS NIH HHS/United States
- R01 AI007289/AI/NIAID NIH HHS/United States
- T32 AI07501/AI/NIAID NIH HHS/United States
- R01 GM054469/GM/NIGMS NIH HHS/United States
- AI07289/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

