Oxidatively damaged DNA in rats exposed by oral gavage to C60 fullerenes and single-walled carbon nanotubes
- PMID: 19479010
- PMCID: PMC2685830
- DOI: 10.1289/ehp.11922
Oxidatively damaged DNA in rats exposed by oral gavage to C60 fullerenes and single-walled carbon nanotubes
Abstract
Background: C60 fullerenes and single-walled carbon nanotubes (SWCNT) are projected to be used in medicine and consumer products with potential human exposure. The hazardous effects of these particles are expected to involve oxidative stress with generation of oxidatively damaged DNA that might be the initiating event in the development of cancer.
Objective: In this study we investigated the effect of a single oral administration of C60 fullerenes and SWCNT.
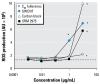
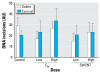
Methods: We measured the level of oxidative damage to DNA as the premutagenic 8-oxo-7,8-dihydro-2'-deoxyguanosine (8-oxodG) in the colon mucosa, liver, and lung of rats after intragastric administration of pristine C60 fullerenes or SWCNT (0.064 or 0.64 mg/kg body weight) suspended in saline solution or corn oil. We investigated the regulation of DNA repair systems toward 8-oxodG in liver and lung tissue.
Results: Both doses of SWCNT increased the levels of 8-oxodG in liver and lung. Administration of C60 fullerenes increased the hepatic level of 8-oxodG, whereas only the high dose generated 8-oxodG in the lung. We detected no effects on 8-oxodG in colon mucosa. Suspension of particles in saline solution or corn oil yielded a similar extent of genotoxicity, whereas corn oil per se generated more genotoxicity than the particles. Although there was increased mRNA expression of 8-oxoguanine DNA glycosylase in the liver of C60 fullerene-treated rats, we found no significant increase in repair activity.
Conclusions: Oral exposure to low doses of C60 fullerenes and SWCNT is associated with elevated levels of 8-oxodG in the liver and lung, which is likely to be caused by a direct genotoxic ability rather than an inhibition of the DNA repair system.
Keywords: DNA damage; DNA repair; cancer; nanoparticle; oxidative stress.
Figures
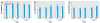
References
-
- Ayres JG, Borm P, Cassee FR, Castranova V, Donaldson K, Ghio A, et al. Evaluating the toxicity of airborne particulate matter and nanoparticles by measuring oxidative stress potential – a workshop report and consensus statement. Inhal Toxicol. 2008;20:75–99. - PubMed
-
- Baker GL, Gupta A, Clark ML, Valenzuela BR, Staska LM, Harbo SJ, et al. Inhalation toxicity and lung toxicokinetics of C60 fullerene nanoparticles and microparticles. Toxicol Sci. 2008;101:122–131. - PubMed
-
- Carr KE, Hazzard RA, Reid S, Hodges GM. The effect of size on uptake of orally administered latex microparticles in the small intestine and transport to mesenteric lymph nodes. Pharm Res. 1996;13:1205–1209. - PubMed
-
- Danielsen PH, Risom L, Wallin H, Autrup H, Vogel U, Loft S, et al. DNA damage in rats after a single oral exposure to diesel exhaust particles. Mutat Res. 2008b;637:49–55. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

